Outline of chemistry
... British William Prout first proposed ordering all the elements by their atomic weight as all atoms had a weight that was an exact multiple of the atomic weight of hydrogen. J. A. R. Newlands devised an early table of elements, which was then developed into the modern periodic table of elements[32] in ...
... British William Prout first proposed ordering all the elements by their atomic weight as all atoms had a weight that was an exact multiple of the atomic weight of hydrogen. J. A. R. Newlands devised an early table of elements, which was then developed into the modern periodic table of elements[32] in ...
Definition - kcpe-kcse
... - react with water to form hydrogen and alkaline solutions; burn in air - al-quili means wood ashes - term dates back to ancient times; people discovered that wood ashes mix with water to produce slippery solutions that can remove grease - one outer electron, by losing this electron they become a ca ...
... - react with water to form hydrogen and alkaline solutions; burn in air - al-quili means wood ashes - term dates back to ancient times; people discovered that wood ashes mix with water to produce slippery solutions that can remove grease - one outer electron, by losing this electron they become a ca ...
APC-Ch.7-Atomic Structure and Periodicity
... Photoelectron Spectroscopy (PES) is a technique that is used to gather information about the electrons in an atom An atom is bombarded with photons. Some of the photons are absorbed and electrons are emitted. the energy is analyzed. Since we can know the energy of the photons, and we know that energ ...
... Photoelectron Spectroscopy (PES) is a technique that is used to gather information about the electrons in an atom An atom is bombarded with photons. Some of the photons are absorbed and electrons are emitted. the energy is analyzed. Since we can know the energy of the photons, and we know that energ ...
Chapter 8 Concepts of Chemical Bonding
... Solve NaF consists of Na+ and F− ions, CsI of Cs+ and I− ions, and CaO of Ca2+ and O2− ions. Because the product Q1Q2 appears in the numerator of Equation 8.4, the lattice energy increases dramatically when the charges increase. Thus, we expect the lattice energy of CaO, which has 2+ and 2− ions, to ...
... Solve NaF consists of Na+ and F− ions, CsI of Cs+ and I− ions, and CaO of Ca2+ and O2− ions. Because the product Q1Q2 appears in the numerator of Equation 8.4, the lattice energy increases dramatically when the charges increase. Thus, we expect the lattice energy of CaO, which has 2+ and 2− ions, to ...
CHEMISTRY-1 CHAPTER 8 CHEMICAL REACTIONS
... Don’t forget about the diatomic elements! (BrINClHOF) For example, Oxygen is O2 as an element. In a compound, it can’t be a diatomic element because it’s not an element anymore, it’s a compound! ...
... Don’t forget about the diatomic elements! (BrINClHOF) For example, Oxygen is O2 as an element. In a compound, it can’t be a diatomic element because it’s not an element anymore, it’s a compound! ...
B) Examples of Avagadro`s Number
... A) Most chemical reactions will continue until one of the reactants is completely used up--then, no more product(s) can be formed B) The reactant that is used up first, and therefore controls how much product is formed, is called the limiting reactant (or limiting reagent) C) The other reactant, in ...
... A) Most chemical reactions will continue until one of the reactants is completely used up--then, no more product(s) can be formed B) The reactant that is used up first, and therefore controls how much product is formed, is called the limiting reactant (or limiting reagent) C) The other reactant, in ...
5.1 - Central Lyon CSD
... Electron Arrangement in Atoms • If this rock were to tumble over, it would end up at a lower height. It would have less energy than before, but its position would be more stable. You will learn that energy and stability play an important role in determining how electrons are configured in an atom. ...
... Electron Arrangement in Atoms • If this rock were to tumble over, it would end up at a lower height. It would have less energy than before, but its position would be more stable. You will learn that energy and stability play an important role in determining how electrons are configured in an atom. ...
Chapter 3 - Stoichiometry
... In chemistry, an amount of matter can be viewed as a specific mass, or a specific volume, or even a specific number of particles. Since atoms and molecules are very small, counting them would be very difficult. But we have to invent a unit such that this standard molecular amount is a specific numbe ...
... In chemistry, an amount of matter can be viewed as a specific mass, or a specific volume, or even a specific number of particles. Since atoms and molecules are very small, counting them would be very difficult. But we have to invent a unit such that this standard molecular amount is a specific numbe ...
Welcome to Chemistry
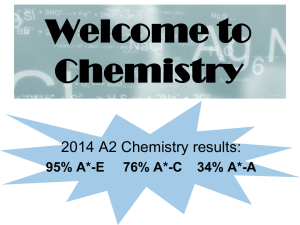
... AS can be sat as a stand alone qualification over 1 year, exams are sat at the end of Y12. 2 written exams each 1 hour and 30 minutes. A level is the full 2 year qualification with all the exams at the end of Y13. 3 written papers each 2 hours. ...
... AS can be sat as a stand alone qualification over 1 year, exams are sat at the end of Y12. 2 written exams each 1 hour and 30 minutes. A level is the full 2 year qualification with all the exams at the end of Y13. 3 written papers each 2 hours. ...
reactions taking place within cells
... 1st law of thermodynamics Energy can’t be created or destroyed, only converted from one form to another Hess’s law H for a reaction is independent of the route it takes provided that the temperatures, pressures and physical states of the reactants and products are the same H of reverse reaction ...
... 1st law of thermodynamics Energy can’t be created or destroyed, only converted from one form to another Hess’s law H for a reaction is independent of the route it takes provided that the temperatures, pressures and physical states of the reactants and products are the same H of reverse reaction ...
- Philsci
... The underlying physical laws necessary for the mathematical theory of a large part of physics and the whole of chemistry are thus completely known, and the difficulty is only that the exact application of these laws leads to equations much too complicated to be soluble. It therefore becomes desirabl ...
... The underlying physical laws necessary for the mathematical theory of a large part of physics and the whole of chemistry are thus completely known, and the difficulty is only that the exact application of these laws leads to equations much too complicated to be soluble. It therefore becomes desirabl ...
File - Science with Mr. Louie
... o Atoms of a given element are identical; atoms of different elements are different o Compounds are formed when atoms of different elements combine with one another. A given compound always has the same relative numbers and types of atoms. o Atoms are not changed in chemical reactions. Designed firs ...
... o Atoms of a given element are identical; atoms of different elements are different o Compounds are formed when atoms of different elements combine with one another. A given compound always has the same relative numbers and types of atoms. o Atoms are not changed in chemical reactions. Designed firs ...
A an electron and an alpha particle B an electron and a proton C a
... empty space ® electrons exist in orbitals outside the nucleus B the atom is a hard sphere ® electrons exist in orbitals outside the nucleus ® most of the atom is empty space C most of the atom is empty space ® electrons exist in orbitals outside the nucleus ® the atom is a hard sphere D most of the ...
... empty space ® electrons exist in orbitals outside the nucleus B the atom is a hard sphere ® electrons exist in orbitals outside the nucleus ® most of the atom is empty space C most of the atom is empty space ® electrons exist in orbitals outside the nucleus ® the atom is a hard sphere D most of the ...
Student Solutions Manual Errata
... bonds are the attractions of oppositely charged ions to one another. We can think of the ions (or the spheres in the diagram) as being separate from, but strongly attracted to, one another. Covalent bonding occurs when two atoms are mutually attracted to a pair (or pairs) of electrons. Because the a ...
... bonds are the attractions of oppositely charged ions to one another. We can think of the ions (or the spheres in the diagram) as being separate from, but strongly attracted to, one another. Covalent bonding occurs when two atoms are mutually attracted to a pair (or pairs) of electrons. Because the a ...
Fall - Physical Chemistry Division
... Graphitic materials are structures based on graphene, a single layer of carbon atoms arranged in a hexagonal honeycomb lattice. Graphitic materials span a wide range of dimensionality starting from 0D fullerenes, to 1D carbon nanotubes (CNTs) and graphene nanoribbons (GNRs), 2D single-layered (or fe ...
... Graphitic materials are structures based on graphene, a single layer of carbon atoms arranged in a hexagonal honeycomb lattice. Graphitic materials span a wide range of dimensionality starting from 0D fullerenes, to 1D carbon nanotubes (CNTs) and graphene nanoribbons (GNRs), 2D single-layered (or fe ...
Supplemental Informaton
... for the structure. i. Given a chemical formula, ABn, A is generally the central atom and B flanks the A atom. i.e., NH3, NCl3, NO2. In these examples, N is central in each structure. ii. H and F are never central atoms even if their elemental symbol is first, i.e., H2O. ...
... for the structure. i. Given a chemical formula, ABn, A is generally the central atom and B flanks the A atom. i.e., NH3, NCl3, NO2. In these examples, N is central in each structure. ii. H and F are never central atoms even if their elemental symbol is first, i.e., H2O. ...
Chemical Changes and Structure Homework Booklet
... 12Mg are two different kinds of magnesium atom. a. What word is used to describe these types of atoms? b. Explain why they can be regarded as atoms of the same element? c. The relative atomic mass of magnesium is 24.3. What does this tell you about the relative amounts of each atom? An atom has atom ...
... 12Mg are two different kinds of magnesium atom. a. What word is used to describe these types of atoms? b. Explain why they can be regarded as atoms of the same element? c. The relative atomic mass of magnesium is 24.3. What does this tell you about the relative amounts of each atom? An atom has atom ...
... • Yes, but it is the empirical formula, not the molecular formula. • An empirical formula only gives the smallest wholenumber ratio of each type of atom in a compound, not the specific number of each type of atom in a molecule. • The molecular formula is always a whole-number multiple of the empiric ...
Chapter 18: Properties of Atoms and the Periodic Table
... Around 400 B.C., Democritus proposed the idea that atoms make up all substances. However, a famous Greek philosopher, Aristotle, disputed Democritus’s theory and proposed that matter was uniform throughout and was not composed of smaller particles. Aristotle’s incorrect theory was accepted for about ...
... Around 400 B.C., Democritus proposed the idea that atoms make up all substances. However, a famous Greek philosopher, Aristotle, disputed Democritus’s theory and proposed that matter was uniform throughout and was not composed of smaller particles. Aristotle’s incorrect theory was accepted for about ...
SUP1111 11 - The Open University
... Oxidation is said to occur when the proportion of oxygen in a compound increases. Conversely, reduction occurs when the proportion of oxygen decreases. The term oxidation is also used more generally to include any reaction in which an atom loses electrons. Conversely, reduction involves a gain of el ...
... Oxidation is said to occur when the proportion of oxygen in a compound increases. Conversely, reduction occurs when the proportion of oxygen decreases. The term oxidation is also used more generally to include any reaction in which an atom loses electrons. Conversely, reduction involves a gain of el ...
Chemical Reactions (Part One)
... Eggs contain a protein called albumen. The protein molecules are long chains of amino acids folded into a ball shape. When eggs are heated, some of the proteins break apart and the molecules unfold. These molecules then join to other nearby protein molecules until they are all linked in a network. 1 ...
... Eggs contain a protein called albumen. The protein molecules are long chains of amino acids folded into a ball shape. When eggs are heated, some of the proteins break apart and the molecules unfold. These molecules then join to other nearby protein molecules until they are all linked in a network. 1 ...
Mole Concept Balancing - The Gurukul Institute
... 5. A flask of 2 dm3 capacity contains O2 at 101.325 kPa and 300 K. The gas pressure is reduced to 0.1 Pa. Assuming ideal behavior, answer the following : (i) What will be the volume of the gas which is left behind? (ii) What amount of O2 nd the corresponding number of molecules re left behind in the ...
... 5. A flask of 2 dm3 capacity contains O2 at 101.325 kPa and 300 K. The gas pressure is reduced to 0.1 Pa. Assuming ideal behavior, answer the following : (i) What will be the volume of the gas which is left behind? (ii) What amount of O2 nd the corresponding number of molecules re left behind in the ...
BalanceEquationsetc
... • A mole is defined as the number of atoms in exactly 12 grams of carbon- 12 • The number is called Avogadro’s Number • One mole of carbon atoms has a mass of 12 grams ...
... • A mole is defined as the number of atoms in exactly 12 grams of carbon- 12 • The number is called Avogadro’s Number • One mole of carbon atoms has a mass of 12 grams ...
Chapter 2 Atoms and Elements Modern Atomic theory
... Atomic Theory- “All matter is composed of atoms grew out of observation and laws” Three important Laws that led to development and acceptance of Atomic Theory Law of conservation of mass Law of definite proportions Law of multiple proportions ...
... Atomic Theory- “All matter is composed of atoms grew out of observation and laws” Three important Laws that led to development and acceptance of Atomic Theory Law of conservation of mass Law of definite proportions Law of multiple proportions ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.