Tutorial 3 - Atomic Theory
... 1. The Prinicipal Quantum Number (n) -positive, whole number (n=1, 2, 3…) -tells you about the size of the orbital, i.e., the distance from the nucleus -tells you about the energy of the orbital; the bigger the number, the higher the energy level -the orbitals form a series of shells (like the layer ...
... 1. The Prinicipal Quantum Number (n) -positive, whole number (n=1, 2, 3…) -tells you about the size of the orbital, i.e., the distance from the nucleus -tells you about the energy of the orbital; the bigger the number, the higher the energy level -the orbitals form a series of shells (like the layer ...
Hein and Arena
... correlating the experimentally observed spectral lines with electron energy levels for the hydrogen atom. ...
... correlating the experimentally observed spectral lines with electron energy levels for the hydrogen atom. ...
Introduction to Atomic Structure - New Jersey Center for Teaching
... According to this four element theory, if one were to just change the proportions of these four "elements", maybe you turn one substance into another - like tin into gold! For instance, they may have thought that tin just needs a little more of the "element" earth in order to be turned into gold. ...
... According to this four element theory, if one were to just change the proportions of these four "elements", maybe you turn one substance into another - like tin into gold! For instance, they may have thought that tin just needs a little more of the "element" earth in order to be turned into gold. ...
Introduction to Atomic Structure - New Jersey Center for Teaching
... Also remember that today we know atoms can be broken down into smaller bits. We also know all atoms of an element are not identical elements found in nature can vary in number of neutrons. However, for the purposes of general Chemistry, Dalton's Postulates are still a pretty reasonable approximation ...
... Also remember that today we know atoms can be broken down into smaller bits. We also know all atoms of an element are not identical elements found in nature can vary in number of neutrons. However, for the purposes of general Chemistry, Dalton's Postulates are still a pretty reasonable approximation ...
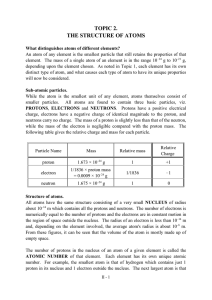
TOPIC 2. THE STRUCTURE OF ATOMS
... As atoms are electrically neutral and the charge on the electron is equal but opposite to that on the proton, there must be identical numbers of electrons and protons in any atom. The electrons are envisaged as being in rapid motion distributed around the nucleus, but never actually being within the ...
... As atoms are electrically neutral and the charge on the electron is equal but opposite to that on the proton, there must be identical numbers of electrons and protons in any atom. The electrons are envisaged as being in rapid motion distributed around the nucleus, but never actually being within the ...
physical setting chemistry
... (1) The gas particles are diatomic. (2) Energy is created when the gas particles collide. (3) There are no attractive forces between the gas particles. (4) The distance between the gas particles is small, compared to their size. ...
... (1) The gas particles are diatomic. (2) Energy is created when the gas particles collide. (3) There are no attractive forces between the gas particles. (4) The distance between the gas particles is small, compared to their size. ...
TOPIC 2. THE STRUCTURE OF ATOMS
... require only a relatively small amount of energy to enter into chemical reactions where the outer 2 electrons are redistributed to atoms of another element, forming cations with a 2+ charge such as Mg2+ from Mg, Ca2+ from Ca, etc. Likewise those elements whose atoms contain 3 more electrons in their ...
... require only a relatively small amount of energy to enter into chemical reactions where the outer 2 electrons are redistributed to atoms of another element, forming cations with a 2+ charge such as Mg2+ from Mg, Ca2+ from Ca, etc. Likewise those elements whose atoms contain 3 more electrons in their ...
Tutorial – Mass mole conversions Std 3e
... With this step, we went from grams to MOLES on our diagram. But we need the moles of O2 not H2 . This is the step that requires the extra MOLES I put into the diagram above. Now it’s time to cancel the units we can and also change moles of H2 to moles of O2 . For that we need to use the mole ratio f ...
... With this step, we went from grams to MOLES on our diagram. But we need the moles of O2 not H2 . This is the step that requires the extra MOLES I put into the diagram above. Now it’s time to cancel the units we can and also change moles of H2 to moles of O2 . For that we need to use the mole ratio f ...
2016
... Future AP Chemistry Student, Welcome to AP Chemistry! I am eagerly anticipating a great year of Chemistry. In order to ensure the best start for everyone next fall, I have prepared a summer assignment that reviews basic chemistry concepts. There is a multitude of tremendous chemistry resources are a ...
... Future AP Chemistry Student, Welcome to AP Chemistry! I am eagerly anticipating a great year of Chemistry. In order to ensure the best start for everyone next fall, I have prepared a summer assignment that reviews basic chemistry concepts. There is a multitude of tremendous chemistry resources are a ...
Atomic Target Practice
... highest atomic mass of the metals studied, gave the largest amount of so-called “back scattering.” Rutherford’s scattering experiments have been described as a “black box” experiment. The properties of the alpha particles, their mass, charge, speed, etc., were at least partially understood. The atom ...
... highest atomic mass of the metals studied, gave the largest amount of so-called “back scattering.” Rutherford’s scattering experiments have been described as a “black box” experiment. The properties of the alpha particles, their mass, charge, speed, etc., were at least partially understood. The atom ...
TOPIC 2. THE STRUCTURE OF ATOMS
... the normal laws of electrostatics would require the electrons to collapse into the nucleus due to the attraction of the protons. Because this does not happen, there must be other laws which govern the behaviour of electrons in atoms. Models to explain this will be presented later in the year as part ...
... the normal laws of electrostatics would require the electrons to collapse into the nucleus due to the attraction of the protons. Because this does not happen, there must be other laws which govern the behaviour of electrons in atoms. Models to explain this will be presented later in the year as part ...
Flavors of the Atom
... Then we lined them up by increasing weight, just like you might organize a poker hand. We gave each element a serial number (atomic number), to indicate it’s place in the sequence of increasing weight. ...
... Then we lined them up by increasing weight, just like you might organize a poker hand. We gave each element a serial number (atomic number), to indicate it’s place in the sequence of increasing weight. ...
Introduction to Computational Chemistry
... flexibility and power of electronic computers, basic principles of classical and quantum mechanics are now implemented in a form which can handle the many-body problems associated with the structure and behavior of complex molecular systems." John A. Pople (November 1997) (Nobel prize for chemistry ...
... flexibility and power of electronic computers, basic principles of classical and quantum mechanics are now implemented in a form which can handle the many-body problems associated with the structure and behavior of complex molecular systems." John A. Pople (November 1997) (Nobel prize for chemistry ...
PPT Oxidation
... reduced and get oxidized. Here are the two halfreactions from the example: Ag+ ---> Ag Cu ---> Cu2+ • The silver is being reduced, its oxidation number going from +1 to zero. The copper's oxidation number went from zero to +2, so it was oxidized in the reaction. In order to figure out the halfreacti ...
... reduced and get oxidized. Here are the two halfreactions from the example: Ag+ ---> Ag Cu ---> Cu2+ • The silver is being reduced, its oxidation number going from +1 to zero. The copper's oxidation number went from zero to +2, so it was oxidized in the reaction. In order to figure out the halfreacti ...
PPT Oxidation
... reduced and get oxidized. Here are the two halfreactions from the example: Ag+ ---> Ag Cu ---> Cu2+ • The silver is being reduced, its oxidation number going from +1 to zero. The copper's oxidation number went from zero to +2, so it was oxidized in the reaction. In order to figure out the halfreacti ...
... reduced and get oxidized. Here are the two halfreactions from the example: Ag+ ---> Ag Cu ---> Cu2+ • The silver is being reduced, its oxidation number going from +1 to zero. The copper's oxidation number went from zero to +2, so it was oxidized in the reaction. In order to figure out the halfreacti ...
Chapter 0 A Very Brief History of Chemistry Multiple Choice Questions
... a. The nebula that formed the earth had elements that were not evenly distributed. b. Winds on the surface of the earth have moved around the heavy and light elements into bands. c. The early earth liquefied, resulting in heavier elements migrating towards the core, and lighter elements towards the ...
... a. The nebula that formed the earth had elements that were not evenly distributed. b. Winds on the surface of the earth have moved around the heavy and light elements into bands. c. The early earth liquefied, resulting in heavier elements migrating towards the core, and lighter elements towards the ...
stationary states in a potential well
... In 1911, the two-and-a half-thousand-year-old philosophical concept of atom turned into a scientific matter when Rutherford’s planetary atomic model emerged from the interpretation of the experimental data on the scattering of α particles [1]. The curious fact that has been noticed while these parti ...
... In 1911, the two-and-a half-thousand-year-old philosophical concept of atom turned into a scientific matter when Rutherford’s planetary atomic model emerged from the interpretation of the experimental data on the scattering of α particles [1]. The curious fact that has been noticed while these parti ...
ppt
... Atom: smallest unit of an element Element: any of more than 100 fundamental substances that consist of atoms of only one kind Molecule: a collection of atoms, bound together. ...
... Atom: smallest unit of an element Element: any of more than 100 fundamental substances that consist of atoms of only one kind Molecule: a collection of atoms, bound together. ...
ch 3_1 atomhist
... • Democritus was a Greek philosopher who thought the universe was made of empty space and tiny bits of stuff. • He believed that the bits of stuff were so small they could no longer be divided into smaller pieces. He called these tiny pieces atomos. • Today an atom is defined as a small particle tha ...
... • Democritus was a Greek philosopher who thought the universe was made of empty space and tiny bits of stuff. • He believed that the bits of stuff were so small they could no longer be divided into smaller pieces. He called these tiny pieces atomos. • Today an atom is defined as a small particle tha ...
Chapter 4 Nomenclature and Chemical Equations
... Depending on the type of bonds present in a compound, different rules are applied to its naming. ...
... Depending on the type of bonds present in a compound, different rules are applied to its naming. ...
Unit 3
... Understand how to correctly write an isotope. Be able to differentiate between alpha (α), beta (β), and gamma (γ) radiation and decay. Be able to draw and describe the basic structure of an atom. Be able to do average atomic mass problems Be able to find wavelength (using Bohr’s model of H ...
... Understand how to correctly write an isotope. Be able to differentiate between alpha (α), beta (β), and gamma (γ) radiation and decay. Be able to draw and describe the basic structure of an atom. Be able to do average atomic mass problems Be able to find wavelength (using Bohr’s model of H ...
1994–PTAS, Inc - mvhs
... (C) The size of the orbital. (D) The shape of the orbital. 6. Atoms and molecules can emit and absorb light (electromagnetic radiation). Describe both of these processes and how they affect the electronic stability of atoms or molecules. ...
... (C) The size of the orbital. (D) The shape of the orbital. 6. Atoms and molecules can emit and absorb light (electromagnetic radiation). Describe both of these processes and how they affect the electronic stability of atoms or molecules. ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.