+2 - Fort Thomas Independent Schools
... Chlorine must be +5 { (+5) + (-6)} = -1 (the ion’s charge) ...
... Chlorine must be +5 { (+5) + (-6)} = -1 (the ion’s charge) ...
Chem 115 POGIL Worksheet - Week 10 Periodic Trends Why? The
... Owing to their relatively low ionization energies, metals tend to form cations, and when they combine with nonmetals they form ionic substances. For example, when metals combine with oxygen they form ionic oxides. 4 Fe(s) + 3 O2 2 Fe2O3(s) Metal oxides tend to dissolve in water to form hydroxide i ...
... Owing to their relatively low ionization energies, metals tend to form cations, and when they combine with nonmetals they form ionic substances. For example, when metals combine with oxygen they form ionic oxides. 4 Fe(s) + 3 O2 2 Fe2O3(s) Metal oxides tend to dissolve in water to form hydroxide i ...
Species Number of protons Number of
... mass number 36, relative abundance 0.337% mass number 38, relative abundance 0.0630% mass number 40, relative abundance 99.6% Calculate the relative atomic mass of argon. ...
... mass number 36, relative abundance 0.337% mass number 38, relative abundance 0.0630% mass number 40, relative abundance 99.6% Calculate the relative atomic mass of argon. ...
Molecular and Empirical Formulas
... • We are told that the experimentally determined molecular mass is 176 amu or g/mol. What is the molecular mass of our empirical formula? • (3*12.011) + (4*1.008) + (3*15.999) = 88.062 g/mol • The molecular mass from our empirical formula is signficantly lower than the experimentally determined valu ...
... • We are told that the experimentally determined molecular mass is 176 amu or g/mol. What is the molecular mass of our empirical formula? • (3*12.011) + (4*1.008) + (3*15.999) = 88.062 g/mol • The molecular mass from our empirical formula is signficantly lower than the experimentally determined valu ...
Problem 5. The Second Law of thermodynamics
... 2. Suppose you detect a signal from a particular 1μm2 area. The probability to have one particle within this area is 0.035. For two particles such probability is (0.035)2 and for three it is equal to (0.035)3 etc. The probability that the detected signal originates from a single Au nanoparticle is: ...
... 2. Suppose you detect a signal from a particular 1μm2 area. The probability to have one particle within this area is 0.035. For two particles such probability is (0.035)2 and for three it is equal to (0.035)3 etc. The probability that the detected signal originates from a single Au nanoparticle is: ...
Solutions (DOC format, upgraded July 20)
... 2. Suppose you detect a signal from a particular 1μm2 area. The probability to have one particle within this area is 0.035. For two particles such probability is (0.035)2 and for three it is equal to (0.035)3 etc. The probability that the detected signal originates from a single Au nanoparticle is: ...
... 2. Suppose you detect a signal from a particular 1μm2 area. The probability to have one particle within this area is 0.035. For two particles such probability is (0.035)2 and for three it is equal to (0.035)3 etc. The probability that the detected signal originates from a single Au nanoparticle is: ...
Problem 5. The Second Law of thermodynamics
... 2. Suppose you detect a signal from a particular 1μm2 area. The probability to have one particle within this area is 0.035. For two particles such probability is (0.035)2 and for three it is equal to (0.035)3 etc. The probability that the detected signal originates from a single Au nanoparticle is: ...
... 2. Suppose you detect a signal from a particular 1μm2 area. The probability to have one particle within this area is 0.035. For two particles such probability is (0.035)2 and for three it is equal to (0.035)3 etc. The probability that the detected signal originates from a single Au nanoparticle is: ...
Chapter 2 Elements and Compounds 2.1 The Structure of the Atom
... As we learned in Chapter 1, compounds are formed when two or more elements are combined chemically in a defined ratio. In this section, we explore covalent compounds, their formulas, their names, and the methods used to represent them. ...
... As we learned in Chapter 1, compounds are formed when two or more elements are combined chemically in a defined ratio. In this section, we explore covalent compounds, their formulas, their names, and the methods used to represent them. ...
TEKS Presentation Properties of Matter
... Example- Water can be changed into hydrogen gas and oxygen gas using an electric current. When water molecules change chemically into hydrogen gas and oxygen gas, we say that a chemical change has occurred. Hydrogen gas and oxygen gas each have a different set of properties. Substances change into d ...
... Example- Water can be changed into hydrogen gas and oxygen gas using an electric current. When water molecules change chemically into hydrogen gas and oxygen gas, we say that a chemical change has occurred. Hydrogen gas and oxygen gas each have a different set of properties. Substances change into d ...
Chemistry - Weird Science With Mrs. Niki
... Just as an atomic orbital belongs to a particular atom, a molecular orbital belongs to a molecule as a whole. A molecular orbital that can be occupied by two electrons of a covalent bond is called a bonding ...
... Just as an atomic orbital belongs to a particular atom, a molecular orbital belongs to a molecule as a whole. A molecular orbital that can be occupied by two electrons of a covalent bond is called a bonding ...
Powerpoint
... physically, because the species of the two half-cells react directly without electrons going through the external circuit. ...
... physically, because the species of the two half-cells react directly without electrons going through the external circuit. ...
File
... 68. The phase diagram for a pure substance is shown above. The solid and gaseous phases of the substances can exist in equilibrium at conditions corresponding to which of the following? A) Point I only B) Point III only C) Any point on the curve from I to II. D) Any point on the curve from II to II ...
... 68. The phase diagram for a pure substance is shown above. The solid and gaseous phases of the substances can exist in equilibrium at conditions corresponding to which of the following? A) Point I only B) Point III only C) Any point on the curve from I to II. D) Any point on the curve from II to II ...
Chapter 2 ATOMS AND ELEMENTS
... • These fill the B-groups (1B through 8B) in the fourth through the seventh periods in the center of the periodic table. • All are metals and 13 of them are in the top 30 elements in terms of abundance in the earth’s crust • Most occur naturally in combination with other elements, but a few, Cu, Ag, ...
... • These fill the B-groups (1B through 8B) in the fourth through the seventh periods in the center of the periodic table. • All are metals and 13 of them are in the top 30 elements in terms of abundance in the earth’s crust • Most occur naturally in combination with other elements, but a few, Cu, Ag, ...
Chemical and Physical Property Unit Test
... a2. What must often be added to increase the speed or ability of two substances to react? A. a bigger container B. adding heat C. more substances D. adding water a3. What kinds of energy are produced by bright fireworks? A. electricity, steam B. motion, gravity C. sound, magnetism D. light, heat b5. ...
... a2. What must often be added to increase the speed or ability of two substances to react? A. a bigger container B. adding heat C. more substances D. adding water a3. What kinds of energy are produced by bright fireworks? A. electricity, steam B. motion, gravity C. sound, magnetism D. light, heat b5. ...
Introductory Chemistry: A Foundation FOURTH EDITION by Steven
... • In a chemical reaction, all the atoms present at the beginning are still present at the end • Therefore the total mass cannot change • Therefore the total mass of the reactants will be the same as the total mass of the products ...
... • In a chemical reaction, all the atoms present at the beginning are still present at the end • Therefore the total mass cannot change • Therefore the total mass of the reactants will be the same as the total mass of the products ...
I. History of the Atomic Theory
... the atom. There were MANY scientists involved in this process. We will focus on only a few. A. JOHN DALTON was an Englishman who was the first to develop and publish a theory about how atoms looked and behaved. He conceived of the atom as a solid sphere, much like a billiard ball. The following are ...
... the atom. There were MANY scientists involved in this process. We will focus on only a few. A. JOHN DALTON was an Englishman who was the first to develop and publish a theory about how atoms looked and behaved. He conceived of the atom as a solid sphere, much like a billiard ball. The following are ...
Chapter 2 Atoms and Elements If You Cut a Piece of Graphite • If you
... © 2014 Pearson Education, Inc. ...
... © 2014 Pearson Education, Inc. ...
Earth’s Materials - Lower Hudson Regional Information Center
... The color of a mineral can be useful, HOWEVER, it can vary due to slight chemical differences The streak is the color of freshly crushed mineral powder and is usually constant. ...
... The color of a mineral can be useful, HOWEVER, it can vary due to slight chemical differences The streak is the color of freshly crushed mineral powder and is usually constant. ...
Biochemistry Assessment
... 1. Graph A. The increase in pressure increases the speed of the reaction. 2. Graph B. The increase in temperature increases the speed of the reaction. 3. Graph C. When temperature and pressure increase, the speed of the reaction increases more than with just temperature or pressure alone. H. Amino A ...
... 1. Graph A. The increase in pressure increases the speed of the reaction. 2. Graph B. The increase in temperature increases the speed of the reaction. 3. Graph C. When temperature and pressure increase, the speed of the reaction increases more than with just temperature or pressure alone. H. Amino A ...
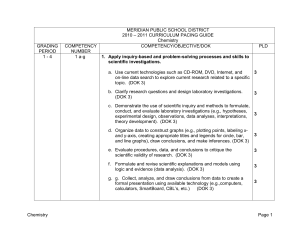
MERIDIAN PUBLIC SCHOOL DISTRICT
... The concept of half-life for a radioactive isotope (e.g., carbon-14 dating) based on the principle that the decay of any individual atom is a random process Page 4 ...
... The concept of half-life for a radioactive isotope (e.g., carbon-14 dating) based on the principle that the decay of any individual atom is a random process Page 4 ...
Final "I Can Statements" Answer Key
... How many L does 4.60 moles of O2 occupy (assuming STP)? 103 L ...
... How many L does 4.60 moles of O2 occupy (assuming STP)? 103 L ...
Atomic structure - Browser Express
... 5. Atoms of different elements combine to form a compound. The numbers of various atoms combined bear a simple whole number ratio to each other. ...
... 5. Atoms of different elements combine to form a compound. The numbers of various atoms combined bear a simple whole number ratio to each other. ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.