FREE Sample Here
... e. The magnetic properties of the core caused the metals to pool into certain areas between the poles on earth. Answer: c Section 0.2 Difficulty Level: medium ...
... e. The magnetic properties of the core caused the metals to pool into certain areas between the poles on earth. Answer: c Section 0.2 Difficulty Level: medium ...
FREE Sample Here
... e. The magnetic properties of the core caused the metals to pool into certain areas between the poles on earth. Answer: c Section 0.2 Difficulty Level: medium ...
... e. The magnetic properties of the core caused the metals to pool into certain areas between the poles on earth. Answer: c Section 0.2 Difficulty Level: medium ...
Atomic Theory Quiz A
... Democritus - Dalton - Thomson - Rutherford - Bohr 2. Put the models of the atom in historical order, oldest to newest: the atom is just an indivisible particle, the atom is a small hard sphere, the atom has a nucleus with electrons flying around outside but not in an organized way, the atom has a nu ...
... Democritus - Dalton - Thomson - Rutherford - Bohr 2. Put the models of the atom in historical order, oldest to newest: the atom is just an indivisible particle, the atom is a small hard sphere, the atom has a nucleus with electrons flying around outside but not in an organized way, the atom has a nu ...
CHAPTER 3 STOICHIOMETRY:
... (c) Is the diagram consistent with the law of conservation of mass? Yes; because there are equal numbers of both N and O atoms in the two boxes ...
... (c) Is the diagram consistent with the law of conservation of mass? Yes; because there are equal numbers of both N and O atoms in the two boxes ...
Atoms, Isotopes, and Ions - Science Take-Out
... 1. Use the information on the periodic table to make a model of a hydrogen atom. Then make a hydrogen ion by removing the electron (blue chip) from the model. Draw your ion model. Use a “+” sign for each proton, an “n” for each neutron and a “–” sign for each electron. ...
... 1. Use the information on the periodic table to make a model of a hydrogen atom. Then make a hydrogen ion by removing the electron (blue chip) from the model. Draw your ion model. Use a “+” sign for each proton, an “n” for each neutron and a “–” sign for each electron. ...
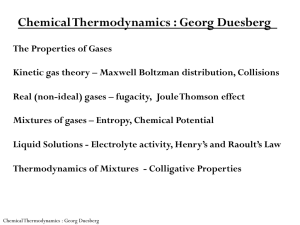
Chemical Thermodynamics : Georg Duesberg
... individual gas atoms/molecules Assumptions: 1) each macroscopic and microscopic particle in motion holds an kinetic energy according to Newton’s law 2) They undergo elastic collisions 3) They are large in number and are randomly distributed 4) They can be treated as points of mass (diameter<< mean f ...
... individual gas atoms/molecules Assumptions: 1) each macroscopic and microscopic particle in motion holds an kinetic energy according to Newton’s law 2) They undergo elastic collisions 3) They are large in number and are randomly distributed 4) They can be treated as points of mass (diameter<< mean f ...
Electrostatics - wths
... the two spheres, charge is allowed to move easily into the other object. The positive particles want to repel each other and get as far away from each other as possible forcing their way throught the conductor. ...
... the two spheres, charge is allowed to move easily into the other object. The positive particles want to repel each other and get as far away from each other as possible forcing their way throught the conductor. ...
Scientific Measurement
... Fusion: change from _________________ to _________________. Solidification: change from _________________ to _________________. Condensation: change from _________________ to _________________. Vaporization: change from _________________ to _________________. _____17. I can state the change of phas ...
... Fusion: change from _________________ to _________________. Solidification: change from _________________ to _________________. Condensation: change from _________________ to _________________. Vaporization: change from _________________ to _________________. _____17. I can state the change of phas ...
Chemistry I Exams and Keys Corrected 2016 Season
... D) 52.5% E) 93.7% 3. Which is/are true for a graph of mass versus volume for a pure substance? I. A straight line is formed with a positive slope. II. A straight line is formed with a negative slope. III. A straight line is formed with a slope of zero. IV. The slope of the line formed is the density ...
... D) 52.5% E) 93.7% 3. Which is/are true for a graph of mass versus volume for a pure substance? I. A straight line is formed with a positive slope. II. A straight line is formed with a negative slope. III. A straight line is formed with a slope of zero. IV. The slope of the line formed is the density ...
Year Review Booklet (optional)
... All atoms of an element are the same Atoms of different elements are different Atoms are indivisible particles Who came up with these ideas? ______________________ He called the ideas, the ___________________________ Theory. ...
... All atoms of an element are the same Atoms of different elements are different Atoms are indivisible particles Who came up with these ideas? ______________________ He called the ideas, the ___________________________ Theory. ...
Preview Sample 1
... 35. The systematic (IUPAC) name of NaClO4 is: A. sodium perchlorate B. sodium chlorate C. sodium hypochlorate D. sodium chloride tetraoxide ...
... 35. The systematic (IUPAC) name of NaClO4 is: A. sodium perchlorate B. sodium chlorate C. sodium hypochlorate D. sodium chloride tetraoxide ...
Sample Chem 111 Final
... 65. What is the average carbon-oxygen bond order in the formate ion ? a) 0 b) 1 c) 1.5 d) 2 e) 2.5 ...
... 65. What is the average carbon-oxygen bond order in the formate ion ? a) 0 b) 1 c) 1.5 d) 2 e) 2.5 ...
ATOMIC STRUCTURE Introduction Modern concept of an
... Democritus studied the nature of matter and the constituents of all the substances. In 1808 John Dalton put forward atomic theory to explain the laws of chemical combination. According to him, an atom is the smallest unit of matter which takes part in a chemical reaction. He considered that atoms ar ...
... Democritus studied the nature of matter and the constituents of all the substances. In 1808 John Dalton put forward atomic theory to explain the laws of chemical combination. According to him, an atom is the smallest unit of matter which takes part in a chemical reaction. He considered that atoms ar ...
The mole and calculations
... Molar Mass: the mass of a substance per 1 mole of its entities (atoms, molecules, ions, or formula units). Units for molar mass are grams/mole. Once again, the periodic table is used to calculate the molar mass (previously we called this molecular mass - it's the same thing!!) 1.) to find the mo ...
... Molar Mass: the mass of a substance per 1 mole of its entities (atoms, molecules, ions, or formula units). Units for molar mass are grams/mole. Once again, the periodic table is used to calculate the molar mass (previously we called this molecular mass - it's the same thing!!) 1.) to find the mo ...
Sub Unit Plan 1 Chem Periodic Table
... II.1 The placement or location of elements on the Periodic Table gives an indication of physical and chemical properties of that element. The elements on the Periodic Table are arranged in order of increasing atomic number. (3.1y) II.2 The number of protons in an atom (atomic number) identifies the ...
... II.1 The placement or location of elements on the Periodic Table gives an indication of physical and chemical properties of that element. The elements on the Periodic Table are arranged in order of increasing atomic number. (3.1y) II.2 The number of protons in an atom (atomic number) identifies the ...
§2 Atomic Structure , A website that gives a good summary of this
... At each sublevel there are 1, 3, 5, 7 orbitals which can contain a maximum 2 electrons according to the P Note the neat mathematical relationships involved in the model for each level n, the thotal number of electrons is 2n2, for each sublevel m there are 2m-1 orbitals where s=1, p=2, d=3, f=4 and f ...
... At each sublevel there are 1, 3, 5, 7 orbitals which can contain a maximum 2 electrons according to the P Note the neat mathematical relationships involved in the model for each level n, the thotal number of electrons is 2n2, for each sublevel m there are 2m-1 orbitals where s=1, p=2, d=3, f=4 and f ...
quantum - kurtniedenzu
... These regions of probability are called orbitals, and this concept replaces that of the electron “orbit”. Instead of electrons orbiting like planets around the sun, Schrodinger’s picture shows an “electron cloud” surrounding the nucleus and doesn’t state anything about the path or position of electr ...
... These regions of probability are called orbitals, and this concept replaces that of the electron “orbit”. Instead of electrons orbiting like planets around the sun, Schrodinger’s picture shows an “electron cloud” surrounding the nucleus and doesn’t state anything about the path or position of electr ...
2nd Semester final review
... A mixture is created when two or more elements or compounds are combined together but do not form a new substance but retain their original properties. 5. Describe each of the diagrams below as either: A) Element B) Compound C) Mixture of elements D) Mixture of compounds E) Mixture of elements and c ...
... A mixture is created when two or more elements or compounds are combined together but do not form a new substance but retain their original properties. 5. Describe each of the diagrams below as either: A) Element B) Compound C) Mixture of elements D) Mixture of compounds E) Mixture of elements and c ...
- skv institute
... hence there is a dipolar interactive attraction between two HCl molecules and such attractive forces are called dipole-dipole forces. 5. What is hydrogen bond enthalpy? 10 to 100 KJmol-1. 6. What is called thermal energy? Thermal energy is the energy of a body arising from motion of its atoms or ...
... hence there is a dipolar interactive attraction between two HCl molecules and such attractive forces are called dipole-dipole forces. 5. What is hydrogen bond enthalpy? 10 to 100 KJmol-1. 6. What is called thermal energy? Thermal energy is the energy of a body arising from motion of its atoms or ...
ALE 23. Balancing Redox Reactions
... The Model Oxidation-reduction or Redox reactions involve the transfer of one or more electrons from one chemical species to another. Redox reactions are involved in the corrosion of metals, the combustion of fuels, the generation of electricity from batteries and many biological processes including ...
... The Model Oxidation-reduction or Redox reactions involve the transfer of one or more electrons from one chemical species to another. Redox reactions are involved in the corrosion of metals, the combustion of fuels, the generation of electricity from batteries and many biological processes including ...
Welcome to 3FF3! Bio
... Multiple non-covalent weak interactions → sum to strong, stable binding non-covalent complexes (e.g. substrate, inhibitor, DNA) ...
... Multiple non-covalent weak interactions → sum to strong, stable binding non-covalent complexes (e.g. substrate, inhibitor, DNA) ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.