BSPH 111 - Refresher Chemistry
... with different numbers of neutrons are isotopes of that element. Isotopes typically exhibit similar chemical behaviour to each other. Isotopes are atoms of the same element with the same number of protons but different number of neutrons. Electrons have such little mass that they exhibit properties ...
... with different numbers of neutrons are isotopes of that element. Isotopes typically exhibit similar chemical behaviour to each other. Isotopes are atoms of the same element with the same number of protons but different number of neutrons. Electrons have such little mass that they exhibit properties ...
AP Chemistry Summer Assignment
... f. Milk turning sour. g. Burning of paper. h. Forming of frost on a cold night. i. Bleaching of hair with hydrogen peroxide. j. A copper wire is hammered flat. ...
... f. Milk turning sour. g. Burning of paper. h. Forming of frost on a cold night. i. Bleaching of hair with hydrogen peroxide. j. A copper wire is hammered flat. ...
CH 2 Worksheet
... theory that such particles existed was supported, much later, by _ (2), who proposed, in his law of __ (3), that matter cannot be created or destroyed. Then (4) proposed, in his law of _____(5), that the ratio of the masses of elements in any given compound is always the same. The law of ____(6), pr ...
... theory that such particles existed was supported, much later, by _ (2), who proposed, in his law of __ (3), that matter cannot be created or destroyed. Then (4) proposed, in his law of _____(5), that the ratio of the masses of elements in any given compound is always the same. The law of ____(6), pr ...
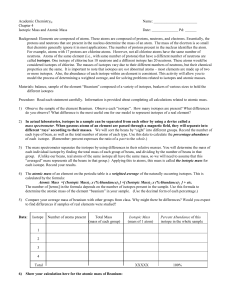
In actual laboratories, isotopes in a sample can be
... neutrons. Atoms of the same element (i.e., with same number of protons) that have a different number of neutrons are called isotopes. One isotope of chlorine has 18 neutrons and a different isotope has 20 neutrons. These atoms would be considered isotopes of chlorine. The masses of isotopes vary due ...
... neutrons. Atoms of the same element (i.e., with same number of protons) that have a different number of neutrons are called isotopes. One isotope of chlorine has 18 neutrons and a different isotope has 20 neutrons. These atoms would be considered isotopes of chlorine. The masses of isotopes vary due ...
CHEM102 Chemistry II Spring 10-11 Mid
... E) The molar mass of a diatomic element is its atomic weight times two. 6) Which statement concerning the mole concept is not true? 6) C A) The molar mass of a metal is its atomic weight expressed in grams. B) One mole of sodium contains the same number of atoms as one mole of carbon. C) One mole of ...
... E) The molar mass of a diatomic element is its atomic weight times two. 6) Which statement concerning the mole concept is not true? 6) C A) The molar mass of a metal is its atomic weight expressed in grams. B) One mole of sodium contains the same number of atoms as one mole of carbon. C) One mole of ...
I. History of the Atomic Theory
... the atom. There were MANY scientists involved in this process. We will focus on only a few. A. JOHN DALTON was an Englishman who was the first to develop and publish a theory about how atoms looked and behaved. He conceived of the atom as a solid sphere, much like a billiard ball. The following are ...
... the atom. There were MANY scientists involved in this process. We will focus on only a few. A. JOHN DALTON was an Englishman who was the first to develop and publish a theory about how atoms looked and behaved. He conceived of the atom as a solid sphere, much like a billiard ball. The following are ...
Atoms and the Periodic Table Atoms and the Periodic Table
... 2. Explain why Dalton’s theory was more successful than Democritus’s theory. 3. List the charge, mass, and location of each of the three subatomic particles found within atoms. 4. Predict how many valence electrons a nitrogen atom has. 5. Explain why oxygen atoms are neutral. 6. Compare an atom’s st ...
... 2. Explain why Dalton’s theory was more successful than Democritus’s theory. 3. List the charge, mass, and location of each of the three subatomic particles found within atoms. 4. Predict how many valence electrons a nitrogen atom has. 5. Explain why oxygen atoms are neutral. 6. Compare an atom’s st ...
Balanced Chemical Equation
... • A Balanced Chemical Equation uses symbolism to represent both the identities and the relative quantities of substances undergoing a chemical (or physical) change. ...
... • A Balanced Chemical Equation uses symbolism to represent both the identities and the relative quantities of substances undergoing a chemical (or physical) change. ...
Chapter 2 - Chemistry
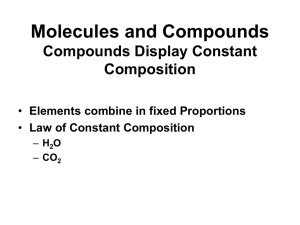
... We can see in the formulas (and models shown) that CO2 has 2 oxygen atoms per carbon atom while CO has 1 oxygen atom per carbon atom. This is reflected in the mass ratio! ...
... We can see in the formulas (and models shown) that CO2 has 2 oxygen atoms per carbon atom while CO has 1 oxygen atom per carbon atom. This is reflected in the mass ratio! ...
Chapter 2 - Chemistry
... We can see in the formulas (and models shown) that CO2 has 2 oxygen atoms per carbon atom while CO has 1 oxygen atom per carbon atom. This is reflected in the mass ratio! All media copyright of their respective owners ...
... We can see in the formulas (and models shown) that CO2 has 2 oxygen atoms per carbon atom while CO has 1 oxygen atom per carbon atom. This is reflected in the mass ratio! All media copyright of their respective owners ...
SED122 - National Open University of Nigeria
... Millikan. The negatively charged particle in matter is the electron, It has negligible mass. The proton is the positively charged particle. It carries the same magnitude of charge as the electron and is very much heavier than the electron. The third particle is the neutron, a neutral particle with a ...
... Millikan. The negatively charged particle in matter is the electron, It has negligible mass. The proton is the positively charged particle. It carries the same magnitude of charge as the electron and is very much heavier than the electron. The third particle is the neutron, a neutral particle with a ...
LEARNING WORKSHEET ON ATOMIC STRUCTURE
... *Metals and nonmetals *Acids and bases *Chemical changes in matter (e.g., rusting [slow oxidation], combustion [fast oxidation], food spoilage) ...
... *Metals and nonmetals *Acids and bases *Chemical changes in matter (e.g., rusting [slow oxidation], combustion [fast oxidation], food spoilage) ...
2013 us national chemistry olympiad
... Structure is correct for most stable. Lone pair of electrons and the least electronegative atom (Cl) are in the equatorial plane at 120 degrees apart with very electronegative F atoms in axial positions, drawing bonding pairs of electrons away from S. ...
... Structure is correct for most stable. Lone pair of electrons and the least electronegative atom (Cl) are in the equatorial plane at 120 degrees apart with very electronegative F atoms in axial positions, drawing bonding pairs of electrons away from S. ...
University of Lusaka
... with different numbers of neutrons are isotopes of that element. Isotopes typically exhibit similar chemical behaviour to each other. Isotopes are atoms of the same element with the same number of protons but different number of neutrons. Electrons have such little mass that they exhibit properties ...
... with different numbers of neutrons are isotopes of that element. Isotopes typically exhibit similar chemical behaviour to each other. Isotopes are atoms of the same element with the same number of protons but different number of neutrons. Electrons have such little mass that they exhibit properties ...
Fall 2012 Chem106 Final Review Name: Test 1 Materials Question
... Question 17. How many Cal. and kJ are in a food item that has 2.5g of fat, 8.5g of carbohydrates, and 3.5g of protein(Fat = 9Cal/g, Prot and Carb = 4 Cal/g)? ...
... Question 17. How many Cal. and kJ are in a food item that has 2.5g of fat, 8.5g of carbohydrates, and 3.5g of protein(Fat = 9Cal/g, Prot and Carb = 4 Cal/g)? ...
What Are Compounds? - Parma School District
... Oxidation Numbers • The charges on the ions in an ionic compound reflect the electron distribution of the compound. • In order to indicate the general distribution of electrons among the bonded atoms in a molecular compound or a polyatomic ion, oxidation numbers are assigned to the atoms composing ...
... Oxidation Numbers • The charges on the ions in an ionic compound reflect the electron distribution of the compound. • In order to indicate the general distribution of electrons among the bonded atoms in a molecular compound or a polyatomic ion, oxidation numbers are assigned to the atoms composing ...
lecture slides file
... Hypothesis – tentative explanation Theory – well-established hypothesis; explanation of general principles of certain phenomena with facts to prove it. - Einstein’s theory of relativity ...
... Hypothesis – tentative explanation Theory – well-established hypothesis; explanation of general principles of certain phenomena with facts to prove it. - Einstein’s theory of relativity ...
Rutherford`s Atomic Model
... particles were deflected at large angles, which could be explained by an atom with a very small, dense, positively-charged nucleus at its center (bottom). ...
... particles were deflected at large angles, which could be explained by an atom with a very small, dense, positively-charged nucleus at its center (bottom). ...
Atomic Structure PowerPoint Presentation
... fundamental particle that everything is made up of? o Is there a universal constant to all matter? ...
... fundamental particle that everything is made up of? o Is there a universal constant to all matter? ...
Bohr model worksheet
... especially that of hydrogen, proposed (1913) by the Danish physicist Niels Bohr. The Bohr model of the atom. On June 19, 1912, Niels Bohr wrote to his brother Harald: "Perhaps I have found out a little about the structure of atoms." I. Setting the Stage This feature is not available right now. Pleas ...
... especially that of hydrogen, proposed (1913) by the Danish physicist Niels Bohr. The Bohr model of the atom. On June 19, 1912, Niels Bohr wrote to his brother Harald: "Perhaps I have found out a little about the structure of atoms." I. Setting the Stage This feature is not available right now. Pleas ...
Unit 2: Structure of Matter Content Outline: History of the Atomic
... B. First to use the term “atom” to describe the basic particle of nature. 1. “atom” means “indivisible” 2. Atom – the smallest particle of an element that still retains the chemical properties of that element. II. John Dalton (1808) A. He was an English schoolteacher. B. He was the first to propose ...
... B. First to use the term “atom” to describe the basic particle of nature. 1. “atom” means “indivisible” 2. Atom – the smallest particle of an element that still retains the chemical properties of that element. II. John Dalton (1808) A. He was an English schoolteacher. B. He was the first to propose ...
Balancing Reaction Equations Oxidation State Reduction
... Balance each of these two reactions using the eight steps we discussed. Assume that the reactions take place in alkaline solution. ...
... Balance each of these two reactions using the eight steps we discussed. Assume that the reactions take place in alkaline solution. ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.