EL Study Notes
... struck a thin sheet of gold foil less than 1µm thick. Alpha particles were known to be positively charged. Predictions based on Thomson’s model of the atom suggested that the alpha particle would be deflected by a few degrees from the straight-ahead direction when they passed through the gold foil. ...
... struck a thin sheet of gold foil less than 1µm thick. Alpha particles were known to be positively charged. Predictions based on Thomson’s model of the atom suggested that the alpha particle would be deflected by a few degrees from the straight-ahead direction when they passed through the gold foil. ...
11 myp covalent bonding
... • Hydrogen and halogens (fluorine, chlorine, bromine and iodine) share only two electrons between the atoms bonded together. A covalent bond consisting of only two shared electrons it is referred to as a single bond. Oxygen and nitrogen however share 4 and 6 electrons respectively. ...
... • Hydrogen and halogens (fluorine, chlorine, bromine and iodine) share only two electrons between the atoms bonded together. A covalent bond consisting of only two shared electrons it is referred to as a single bond. Oxygen and nitrogen however share 4 and 6 electrons respectively. ...
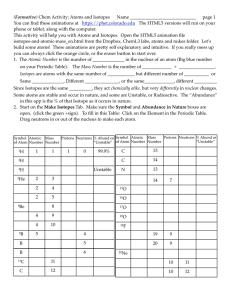
Calculating the number of Protons, Neutrons, and Electrons.
... The Atom • Is about….. – Anything that has matter/mass or takes up space. – Made up of three subatomic particles (protons, neutrons, and electrons) ...
... The Atom • Is about….. – Anything that has matter/mass or takes up space. – Made up of three subatomic particles (protons, neutrons, and electrons) ...
Atomic Physics Sections 9.1-9.7
... elements that compose it. For example, when water is broken down by electrolysis into oxygen and hydrogen, the mass ratio is always 8 to 1. ...
... elements that compose it. For example, when water is broken down by electrolysis into oxygen and hydrogen, the mass ratio is always 8 to 1. ...
Name Honors Chemistry ___/___/___ Subatomic Particles Atomic
... and electrons have a -1 charge, in a neutral atom the number of protons is equal to the number of electrons. Thus, in a neutral atom, the atomic number also indicates the number of electrons in an atom. (For now, assume all atoms are neutral. We will discuss charged atoms later this year.) Summary: ...
... and electrons have a -1 charge, in a neutral atom the number of protons is equal to the number of electrons. Thus, in a neutral atom, the atomic number also indicates the number of electrons in an atom. (For now, assume all atoms are neutral. We will discuss charged atoms later this year.) Summary: ...
Final Exam Review 2010 UbD
... 40. What are the 2 conclusions Rutherford made about the structure of the atom after his Gold Foil Experiment? ___________________________________________________________________________ ______________________________________________________________________________________ 41. What is the mass of t ...
... 40. What are the 2 conclusions Rutherford made about the structure of the atom after his Gold Foil Experiment? ___________________________________________________________________________ ______________________________________________________________________________________ 41. What is the mass of t ...
Atoms and nukes packet 2016
... 3. So Write this General Rule: If there is only one stable isotope of an element, its Average Atomic mass is : ...
... 3. So Write this General Rule: If there is only one stable isotope of an element, its Average Atomic mass is : ...
H 2
... 2. Single Replacement Reactions: The reactants are an element and a compound and the products are a different element and compound. A metallic element will replace the positive part of a compound or a nonmetallic element will replace the negative part of a compound. 3. Synthesis (Combination) Reacti ...
... 2. Single Replacement Reactions: The reactants are an element and a compound and the products are a different element and compound. A metallic element will replace the positive part of a compound or a nonmetallic element will replace the negative part of a compound. 3. Synthesis (Combination) Reacti ...
Chemistry Workbook 1-1A
... charged electrons reside in the atom surrounded by a diffuse, continuous medium of positive charge (like plums in a pudding, or for a more modern analogy, like chocolate chips in a cookie). (a) In 1911 Earnest Rutherford carried out an experiment that changed the way we view the atom. Briefly descri ...
... charged electrons reside in the atom surrounded by a diffuse, continuous medium of positive charge (like plums in a pudding, or for a more modern analogy, like chocolate chips in a cookie). (a) In 1911 Earnest Rutherford carried out an experiment that changed the way we view the atom. Briefly descri ...
W1 WORKSHOP ON STOICHIOMETRY
... Q16. Write the ionic equations for the reactions that occur when solid sodium carbonate and solid calcium chloride dissolve in water. Also write the ionic equation for the precipitation of calcium carbonate resulting from mixing the two solutions. Dissolution of sodium carbonate ...
... Q16. Write the ionic equations for the reactions that occur when solid sodium carbonate and solid calcium chloride dissolve in water. Also write the ionic equation for the precipitation of calcium carbonate resulting from mixing the two solutions. Dissolution of sodium carbonate ...
unit_k_reading_notes
... already seen—it’s composition stoichiometry, which is the study of mass relationships of elements in compounds. Examples of this include calculating percentage composition, and determination of empirical and molecular formulas. The second one is reaction stoichiometry, which deals with the mass, mol ...
... already seen—it’s composition stoichiometry, which is the study of mass relationships of elements in compounds. Examples of this include calculating percentage composition, and determination of empirical and molecular formulas. The second one is reaction stoichiometry, which deals with the mass, mol ...
Chapter 5 - Valencia College
... An element is composed of tiny, indivisible, indestructible particles called atoms. 1. All atoms of an element are identical and have the same properties. 2. Atoms of different elements combine to form compounds. 3. Compounds contain atoms in small whole number ratios. 4. Atoms can combine in more t ...
... An element is composed of tiny, indivisible, indestructible particles called atoms. 1. All atoms of an element are identical and have the same properties. 2. Atoms of different elements combine to form compounds. 3. Compounds contain atoms in small whole number ratios. 4. Atoms can combine in more t ...
STUDY GUIDE
... cotton, and wood are all composed of polymers made by living organisms. Polymers are large molecules—natural or synthetic—made up of many monomers linked together. Homopolymers are polymers made of only a single type of monomer. Copolymers are polymers made of two or more types of monomers. Addition ...
... cotton, and wood are all composed of polymers made by living organisms. Polymers are large molecules—natural or synthetic—made up of many monomers linked together. Homopolymers are polymers made of only a single type of monomer. Copolymers are polymers made of two or more types of monomers. Addition ...
Chapter 3 Mass Relationships in Chemical Reactions 1
... When 1.00 metric ton (1 × 103 kg) of trona is decomposed, 0.74 metric ton of Na2CO3 is recovered. What is the percent yield of this reaction? (b) 43% (c) 22% (d) 83% (a) 93%3 104. When octane (C8H18) is burned in a particular internal combustion engine, the yield of products (carbon dioxide and wate ...
... When 1.00 metric ton (1 × 103 kg) of trona is decomposed, 0.74 metric ton of Na2CO3 is recovered. What is the percent yield of this reaction? (b) 43% (c) 22% (d) 83% (a) 93%3 104. When octane (C8H18) is burned in a particular internal combustion engine, the yield of products (carbon dioxide and wate ...
Welcome`to`AP`Chemistry!
... quantity, just as 9 and 4, and is, therefore, a significant number. A zero between any of the other digits in a number is a significant figure. Zero at the Front of a Number. In reading the measurement 0.46 cm, the zero does not represent a measured quantity, but merely locates the decimal point. It ...
... quantity, just as 9 and 4, and is, therefore, a significant number. A zero between any of the other digits in a number is a significant figure. Zero at the Front of a Number. In reading the measurement 0.46 cm, the zero does not represent a measured quantity, but merely locates the decimal point. It ...
Q1. This question is about the structure of atoms. (a) Choose words
... The chemical formula of ammonia is NH3. This shows that there is one atom of .......................................... and three atoms of .................................. in each ......................................... of ammonia. These atoms are joined by bonds that are formed by sharing pairs ...
... The chemical formula of ammonia is NH3. This shows that there is one atom of .......................................... and three atoms of .................................. in each ......................................... of ammonia. These atoms are joined by bonds that are formed by sharing pairs ...
4 Structure of The Atom
... electrons are embedded in a positive sphere made from the protons. 6. Rutherford’s atomic model proposed that a very, very small nucleus is present inside the atom and the electrons revolve around it in fixed orbits or shells, much like the planets revolve around the Sun. The stability of the ato ...
... electrons are embedded in a positive sphere made from the protons. 6. Rutherford’s atomic model proposed that a very, very small nucleus is present inside the atom and the electrons revolve around it in fixed orbits or shells, much like the planets revolve around the Sun. The stability of the ato ...
AP Chemistry - School Webmasters
... Future AP Chemistry Student, Welcome to AP Chemistry. In order to ensure the best start for everyone next fall, I have prepared a summer assignment that reviews basic chemistry concepts some of which you may have forgotten you learned. For those topics you need help with there are a multitude of tre ...
... Future AP Chemistry Student, Welcome to AP Chemistry. In order to ensure the best start for everyone next fall, I have prepared a summer assignment that reviews basic chemistry concepts some of which you may have forgotten you learned. For those topics you need help with there are a multitude of tre ...
Chapter 17: An Introduction to Organic Chemistry, Biochemistry, and
... Although Lewis structures are useful for describing the bonding within molecules, they can be time consuming to draw, and they do not show the spatial relationships of the atoms well. For example, the Lewis structure of butyl ethyl ether seems to indicate that the bond angles around each carbon atom ...
... Although Lewis structures are useful for describing the bonding within molecules, they can be time consuming to draw, and they do not show the spatial relationships of the atoms well. For example, the Lewis structure of butyl ethyl ether seems to indicate that the bond angles around each carbon atom ...
8.3 Bonding Theories
... 8.3 Bonding Theories > Glossary Terms 5 tetrahedral angle: a bond angle of 109.5° that results when a central atom forms four bonds directed toward the center of a regular tetrahedron 6 VSEPR theory: valence-shell electron-pair repulsion theory; because electron pairs repel, molecules adjust their ...
... 8.3 Bonding Theories > Glossary Terms 5 tetrahedral angle: a bond angle of 109.5° that results when a central atom forms four bonds directed toward the center of a regular tetrahedron 6 VSEPR theory: valence-shell electron-pair repulsion theory; because electron pairs repel, molecules adjust their ...
Molecular geometry
... Valence bond theory (VB): An advanced model of chemical bonding in which electrons reside in quantum-mechanical orbitals localized on individual atoms that are a hybridized blend of standard atomic orbitals; chemical bonds result from an overlap of these orbitals. Molecular orbital theory (MO): ...
... Valence bond theory (VB): An advanced model of chemical bonding in which electrons reside in quantum-mechanical orbitals localized on individual atoms that are a hybridized blend of standard atomic orbitals; chemical bonds result from an overlap of these orbitals. Molecular orbital theory (MO): ...
chem10chp7spr08
... Predict the product – has already been given, but we’ll learn how to do this later Write the correct chemical formulas – keep working on this __Al(s) + __Cl2(g) __AlCl3(s) Not mass balanced Balance equation using the correct stoich coefficients 1 Al & 2 Cl 1 Al & 3 Cl 2 Cl vs. 3 Cl: Find least c ...
... Predict the product – has already been given, but we’ll learn how to do this later Write the correct chemical formulas – keep working on this __Al(s) + __Cl2(g) __AlCl3(s) Not mass balanced Balance equation using the correct stoich coefficients 1 Al & 2 Cl 1 Al & 3 Cl 2 Cl vs. 3 Cl: Find least c ...
NC Exam Questions - Rosshall Academy
... 4. Rum flavouring is based on the compound with the formula shown. ...
... 4. Rum flavouring is based on the compound with the formula shown. ...
Power Point Presentation
... Greek Four-element View of Matter Each element is a combination of two properties: Fire = hot + dry Earth = cold + dry Water = cold + wet Air = hot + wet Aristotle’s definition of an element: Let us define the Element in bodies as that into which other bodies may be analyzed, which are present in t ...
... Greek Four-element View of Matter Each element is a combination of two properties: Fire = hot + dry Earth = cold + dry Water = cold + wet Air = hot + wet Aristotle’s definition of an element: Let us define the Element in bodies as that into which other bodies may be analyzed, which are present in t ...
Honors Chemistry Unit 02
... quite), but atoms of different elements have different masses. – Atoms combine in simple, whole-number ratios to form compounds. – Atoms of one element cannot change into atoms of another element (not quite). In a chemical reaction, atoms change the way they are bound together with other atoms to fo ...
... quite), but atoms of different elements have different masses. – Atoms combine in simple, whole-number ratios to form compounds. – Atoms of one element cannot change into atoms of another element (not quite). In a chemical reaction, atoms change the way they are bound together with other atoms to fo ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.