p,d,f
... generate hypotheses related to the molecular design of compounds for which data are not supplied. [See SP 3.1, 5.1; Essential knowledge 1.C.1] Learning objective 1.12 The student is able to explain why a given set of data suggests, or does not suggest, the need to refine the atomic model from a clas ...
... generate hypotheses related to the molecular design of compounds for which data are not supplied. [See SP 3.1, 5.1; Essential knowledge 1.C.1] Learning objective 1.12 The student is able to explain why a given set of data suggests, or does not suggest, the need to refine the atomic model from a clas ...
physical change
... Each molecule of a compound contains two or more elements that are chemically combined. ...
... Each molecule of a compound contains two or more elements that are chemically combined. ...
Lecture 1 - Алтайский государственный технический
... 4. Compounds are formed when atoms of more than one element combine; a given compound always has the same relative number and kind of atoms. Atoms are the basic building blocks of matter; they are the smallest units of an element: an element is composed of only one kind of atom; in compounds the ato ...
... 4. Compounds are formed when atoms of more than one element combine; a given compound always has the same relative number and kind of atoms. Atoms are the basic building blocks of matter; they are the smallest units of an element: an element is composed of only one kind of atom; in compounds the ato ...
DOC
... moved along the same orbit. But now, s electrons, because their antiparallel spin fields alter each other's courses slightly, are regarded as occupying separate orbits. Shell number 2 can have a total of eight electrons which are divided into two subshells. Two a electrons form a spherical configura ...
... moved along the same orbit. But now, s electrons, because their antiparallel spin fields alter each other's courses slightly, are regarded as occupying separate orbits. Shell number 2 can have a total of eight electrons which are divided into two subshells. Two a electrons form a spherical configura ...
Chapter 2
... The mass of 1 atom of carbon-12 is defined to be 12 amu. Atomic Mass: The weighted average of the isotopic masses of the element’s naturally occurring isotopes ...
... The mass of 1 atom of carbon-12 is defined to be 12 amu. Atomic Mass: The weighted average of the isotopic masses of the element’s naturally occurring isotopes ...
110 exam i material
... A. Atoms are the smallest units/particles that can exist that will have the characteristics of the element. ...
... A. Atoms are the smallest units/particles that can exist that will have the characteristics of the element. ...
Sample pages 1 PDF
... In 1869, a Russian chemist named Dmitri I. Mendeleev (1834–1907) discovered that elements exhibit a repeating pattern of properties when organized in order of increasing atomic mass. He called this observation the periodic law. Mendeleyev arranged the elements in different ways to determine if a rel ...
... In 1869, a Russian chemist named Dmitri I. Mendeleev (1834–1907) discovered that elements exhibit a repeating pattern of properties when organized in order of increasing atomic mass. He called this observation the periodic law. Mendeleyev arranged the elements in different ways to determine if a rel ...
Electron configuration
... element and between different elements; neither of these is true (although they are approximately true enough for the principle to be useful). It considers atomic orbitals as "boxes" of fixed energy into which can be placed two electrons and no more. However the energy of an electron "in" an atomic ...
... element and between different elements; neither of these is true (although they are approximately true enough for the principle to be useful). It considers atomic orbitals as "boxes" of fixed energy into which can be placed two electrons and no more. However the energy of an electron "in" an atomic ...
File - Mr Weng`s IB Chemistry
... • Masses of atoms are compared on a scale relative to 12C and are expressed as relative atomic mass (Ar) and relative formula/molecular mass (Mr). • Molar mass (M) has the units g mol-1. • The empirical formula and molecular formula of a compound give the simplest ratio and the actual number of atom ...
... • Masses of atoms are compared on a scale relative to 12C and are expressed as relative atomic mass (Ar) and relative formula/molecular mass (Mr). • Molar mass (M) has the units g mol-1. • The empirical formula and molecular formula of a compound give the simplest ratio and the actual number of atom ...
Chapter 4: The Structure of the Atom
... copper atoms (equal in number to the world’s population) side by side would result in a line of copper atoms less than one meter long. You might think that because atoms are so small there would be no way to actually see them. However, an instrument called the scanning tunneling microscope allows in ...
... copper atoms (equal in number to the world’s population) side by side would result in a line of copper atoms less than one meter long. You might think that because atoms are so small there would be no way to actually see them. However, an instrument called the scanning tunneling microscope allows in ...
atoms - ChilhowieMiddleSchool
... History of the Atom John Dalton Matter is made up of atoms Atoms cannot be divided. All atoms of the same element are alike. Different elements are made of different atoms. ...
... History of the Atom John Dalton Matter is made up of atoms Atoms cannot be divided. All atoms of the same element are alike. Different elements are made of different atoms. ...
Chapter 4 What Are Atoms?
... • Our understanding of atoms required many centuries. • The idea of an atom—which means “unable to be divided”—dates back to the Greek philosopher Democritus, who lived in the fourth century BCE. • John Dalton developed an atomic theory in 1808. • Like Democritus, Dalton proposed that atoms could no ...
... • Our understanding of atoms required many centuries. • The idea of an atom—which means “unable to be divided”—dates back to the Greek philosopher Democritus, who lived in the fourth century BCE. • John Dalton developed an atomic theory in 1808. • Like Democritus, Dalton proposed that atoms could no ...
Nucleon number
... 2. All the following statements are true EXCEPT A Nucleus is the positively charged centre of an atom B Protons number indicates the number of protons in an atom C Isotopes are atoms of the same element but with different nucleon number D Nucleon number is the total number of electrons and protons i ...
... 2. All the following statements are true EXCEPT A Nucleus is the positively charged centre of an atom B Protons number indicates the number of protons in an atom C Isotopes are atoms of the same element but with different nucleon number D Nucleon number is the total number of electrons and protons i ...
Noble Prize In Physics
... In chemistry and physics, atomic theory is a theory of the nature of matter, which states that matter is composed of discrete units called atoms, as opposed to obsolete beliefs that matter could be divided into any arbitrarily small quantity. Or, in a nutshell, the idea that all things are made of a ...
... In chemistry and physics, atomic theory is a theory of the nature of matter, which states that matter is composed of discrete units called atoms, as opposed to obsolete beliefs that matter could be divided into any arbitrarily small quantity. Or, in a nutshell, the idea that all things are made of a ...
lesson 4.notebook
... Given the following element, Nickel answer these questions: 1. How many protons does the nickel atom have? 2. What is the family/series name? 3. Is it a metal or nonmetal? 4. Give the period and group number in that order ...
... Given the following element, Nickel answer these questions: 1. How many protons does the nickel atom have? 2. What is the family/series name? 3. Is it a metal or nonmetal? 4. Give the period and group number in that order ...
Chapter 07 and 08 Chemical Bonding and Molecular
... • Pure substance • Made of 2 or more elements in a definite proportion by mass • Physically and chemically different from the elements that make up the compound • All elements (except Noble gases) react to gain a stable octet. (duet-for H through B) • Compounds form to gain a stable valence shell wh ...
... • Pure substance • Made of 2 or more elements in a definite proportion by mass • Physically and chemically different from the elements that make up the compound • All elements (except Noble gases) react to gain a stable octet. (duet-for H through B) • Compounds form to gain a stable valence shell wh ...
Chemistry - Ysgol Bro Pedr
... electron of a particular energy. These regions are called orbitals. Shells are split into subshells, which contain orbitals of the same type. Each orbital can contain a maximum of two electrons. These electrons both have a negative charge and, in order to stop them repulsing each other totally, they ...
... electron of a particular energy. These regions are called orbitals. Shells are split into subshells, which contain orbitals of the same type. Each orbital can contain a maximum of two electrons. These electrons both have a negative charge and, in order to stop them repulsing each other totally, they ...
Reaction Stoichiometry
... We cannot simply add the total moles of all the reactants to decide which reactant mixture makes the most product. We must always think about how much product can be formed by using what we are given, and the ratio in the balanced equation. ...
... We cannot simply add the total moles of all the reactants to decide which reactant mixture makes the most product. We must always think about how much product can be formed by using what we are given, and the ratio in the balanced equation. ...
AP Chemistry Summer Assignment 2016
... Directions: Having completed a first course in chemistry, and having elected to take Advanced Placement Chemistry in the fall, you are asked to be responsible for many of the topics presented in the first course. In order to enhance the presentation of new concepts, you are asked to review the follo ...
... Directions: Having completed a first course in chemistry, and having elected to take Advanced Placement Chemistry in the fall, you are asked to be responsible for many of the topics presented in the first course. In order to enhance the presentation of new concepts, you are asked to review the follo ...
CHEMISTRY 123-07 Midterm #1 – Answer key October 14, 2010
... Answer: Examine separately the amounts of a product generated from KO2 and H2O individually. Since the next question is about the quantity of O2, let us choose O2 as the product to study: 1. Mol amount of O2 from KO2: 0.25 mol KO2 x (3 mol O2/4 mol KO2) = 0.19 mol O2 2. Mol amount of O2 from H2O: 0. ...
... Answer: Examine separately the amounts of a product generated from KO2 and H2O individually. Since the next question is about the quantity of O2, let us choose O2 as the product to study: 1. Mol amount of O2 from KO2: 0.25 mol KO2 x (3 mol O2/4 mol KO2) = 0.19 mol O2 2. Mol amount of O2 from H2O: 0. ...
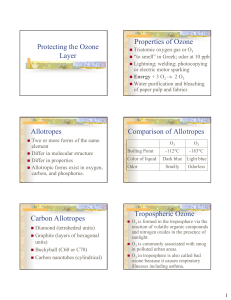
Protecting the Ozone Layer Properties of Ozone Allotropes
... Specific atom Isotopes have different mass numbers 2.5 Your Turn, page 51 ...
... Specific atom Isotopes have different mass numbers 2.5 Your Turn, page 51 ...
4.1 Studying Atoms
... The philosopher Democritus believed that all matter consisted of extremely small particles that could not be divided. He called these particles atoms from the Greek word atomos, which means “uncut” or “indivisible.” He thought there were different types of atoms with specific sets of properties. The ...
... The philosopher Democritus believed that all matter consisted of extremely small particles that could not be divided. He called these particles atoms from the Greek word atomos, which means “uncut” or “indivisible.” He thought there were different types of atoms with specific sets of properties. The ...
2.6 M - Thierry Karsenti
... This module in organic chemistry is designed to prepare students that wish to become teachers : Student teachers must have knowledge of the key concepts and classification tools of organic chemistry that include functional groups of hydrocarbons,alcohols and ethers,aldehydes and ketones,alkyl halide ...
... This module in organic chemistry is designed to prepare students that wish to become teachers : Student teachers must have knowledge of the key concepts and classification tools of organic chemistry that include functional groups of hydrocarbons,alcohols and ethers,aldehydes and ketones,alkyl halide ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.