Topic_4
... The number of moles of a gas (n) can be determined if the pressure (P), temperature (T), and volume (V) of the gas sample are known, using the constant R according to the following equation: PV = nRT An empirical formula shows the smallest whole number ratio of elements in a compound. Ionic solids a ...
... The number of moles of a gas (n) can be determined if the pressure (P), temperature (T), and volume (V) of the gas sample are known, using the constant R according to the following equation: PV = nRT An empirical formula shows the smallest whole number ratio of elements in a compound. Ionic solids a ...
Answers - U of L Class Index
... pass through biological tissues, and they do most of their damage just as they are about to stop. By choosing their energy correctly, it is therefore possible to target a tumor at a very specific depth, causing much less damage to intervening tissues. In one particular operation, protons with a kine ...
... pass through biological tissues, and they do most of their damage just as they are about to stop. By choosing their energy correctly, it is therefore possible to target a tumor at a very specific depth, causing much less damage to intervening tissues. In one particular operation, protons with a kine ...
Wizard Test Maker
... electricity. This element could be (1) H (3) S (2) He (4) Sn 6600 An element that is malleable and a good conductor of heat and electricity could have an atomic number of ...
... electricity. This element could be (1) H (3) S (2) He (4) Sn 6600 An element that is malleable and a good conductor of heat and electricity could have an atomic number of ...
Classification of Matter
... If a pure substance cannot be decomposed into something else, then the substance is an element. There are 114 elements known. Each element is given a unique chemical symbol (one or two letters). Elements are building blocks of matter. The earth’s crust consists of 5 main elements. The human body con ...
... If a pure substance cannot be decomposed into something else, then the substance is an element. There are 114 elements known. Each element is given a unique chemical symbol (one or two letters). Elements are building blocks of matter. The earth’s crust consists of 5 main elements. The human body con ...
Chemical Bonding
... Explaining the Properties of Ionic Compounds Ionic compounds have similar properties: They are solids at SATP with high melting points, and they are electrolytes. We can hypothesize that these properties might be the result of the bonds formed between the ions, holding them firmly in a rigid structu ...
... Explaining the Properties of Ionic Compounds Ionic compounds have similar properties: They are solids at SATP with high melting points, and they are electrolytes. We can hypothesize that these properties might be the result of the bonds formed between the ions, holding them firmly in a rigid structu ...
Chem 107 - Hughbanks Exam 1
... (10) (12 points) Cryolite, Na3AlF6, is used in the commercial production of aluminum metal from bauxite ore (Al2O3). Cryolite itself is produced by the following (unbalanced) reaction: ...
... (10) (12 points) Cryolite, Na3AlF6, is used in the commercial production of aluminum metal from bauxite ore (Al2O3). Cryolite itself is produced by the following (unbalanced) reaction: ...
4/page
... the state is usually called the phase, such as “the liquid phase” kinetic (motion) energy of particles increases with increasing temperature T Feb. 3, 2006 ...
... the state is usually called the phase, such as “the liquid phase” kinetic (motion) energy of particles increases with increasing temperature T Feb. 3, 2006 ...
Document
... from the lowest possible energy to the highest energy. • Hund’s Rule In orbitals of EQUAL ENERGY (p, d, and f), place one electron in each orbital before making any pairs. All single electrons must spin the same way • Pauli Exclusion Principle says that no two electrons within an atom (or ion) can h ...
... from the lowest possible energy to the highest energy. • Hund’s Rule In orbitals of EQUAL ENERGY (p, d, and f), place one electron in each orbital before making any pairs. All single electrons must spin the same way • Pauli Exclusion Principle says that no two electrons within an atom (or ion) can h ...
AJAY PARMAR GROUP TUITION
... Rutherford produced alpha () rays from radioactive element polonium (Po). These rays were incident from one side on the foil (0.004 mm thick) of gold. Observations of Rutherford’s experiment: Most of the rays passed straight through the foil. Very few of them did not pass through, but reflected ...
... Rutherford produced alpha () rays from radioactive element polonium (Po). These rays were incident from one side on the foil (0.004 mm thick) of gold. Observations of Rutherford’s experiment: Most of the rays passed straight through the foil. Very few of them did not pass through, but reflected ...
Chemistry - NIC Karnataka
... Electronegativity. valence – periodicity of valence or oxidation states (s and p block elements). ...
... Electronegativity. valence – periodicity of valence or oxidation states (s and p block elements). ...
Spectrum05
... 2C2H2 + 5 O2 4CO2 + 2 H2O If 3.84 moles of C2H2 are burned, how many moles of O2 are needed? How many moles of C2H2 are needed to produce 8.95 mole of H2O? If 2.47 moles of C2H2 are burned, how many moles of CO2 are formed? ...
... 2C2H2 + 5 O2 4CO2 + 2 H2O If 3.84 moles of C2H2 are burned, how many moles of O2 are needed? How many moles of C2H2 are needed to produce 8.95 mole of H2O? If 2.47 moles of C2H2 are burned, how many moles of CO2 are formed? ...
Research Papers-Quantum Theory / Particle Physics/Download/6583
... The shell Group of the third range (the third Group) has three shells, in one of which there may be filled all three orbits, the first of which has to be filled with two encountering electrons, the second – with two encountering dyads, and the third – with two encountering triads. The half total amo ...
... The shell Group of the third range (the third Group) has three shells, in one of which there may be filled all three orbits, the first of which has to be filled with two encountering electrons, the second – with two encountering dyads, and the third – with two encountering triads. The half total amo ...
Slide 1
... • Coined in 1894, derived from the term electric, whose ultimate origin is from the Greek word meaning “amber” • Negatively charged particles that orbit around the outside of the nucleus. • The sharing or exchange of electrons between atoms forms chemical bonds, which is how new molecules and compou ...
... • Coined in 1894, derived from the term electric, whose ultimate origin is from the Greek word meaning “amber” • Negatively charged particles that orbit around the outside of the nucleus. • The sharing or exchange of electrons between atoms forms chemical bonds, which is how new molecules and compou ...
1 - Montville.net
... AP Chemistry can be considered as the second year of a two year program. The topics covered at the beginning of the year are mostly review from first year chemistry so we will move very quickly through this material. The purpose of this assignment is to review some of the material you learned last y ...
... AP Chemistry can be considered as the second year of a two year program. The topics covered at the beginning of the year are mostly review from first year chemistry so we will move very quickly through this material. The purpose of this assignment is to review some of the material you learned last y ...
Name - TeacherWeb
... The elements in Group 18 are known as the noble gases. They do not usually form compounds because they do not like to gain, lose, or share electrons. All of the noble gases exist in the Earth’s atmosphere, but only in small amounts. ...
... The elements in Group 18 are known as the noble gases. They do not usually form compounds because they do not like to gain, lose, or share electrons. All of the noble gases exist in the Earth’s atmosphere, but only in small amounts. ...
ESO - ENCIGA
... about them and to formulate laws and principles based on these facts. The organized knowledge that is derived from scientific studies is continuously tested by subsequent investigation and can be modified by its results. Science does not give statements of absolute eternal truth, it only provides th ...
... about them and to formulate laws and principles based on these facts. The organized knowledge that is derived from scientific studies is continuously tested by subsequent investigation and can be modified by its results. Science does not give statements of absolute eternal truth, it only provides th ...
Properties of Atoms - Bremen High School District 228
... philosopher, Aristotle, disputed Democritus’s theory and proposed that matter was uniform throughout and was not composed of smaller particles. Aristotle’s incorrect theory was accepted for about 2,000 years. In the 1800s, John Dalton, an English scientist, was able to offer proof that atoms exist. ...
... philosopher, Aristotle, disputed Democritus’s theory and proposed that matter was uniform throughout and was not composed of smaller particles. Aristotle’s incorrect theory was accepted for about 2,000 years. In the 1800s, John Dalton, an English scientist, was able to offer proof that atoms exist. ...
Periodic Law
... electron's charge. The periodic law correlates very closely with the electronic structures of atoms. The law is demonstrated most clearly through a tabular arrangement that groups elements with similar properties together in an array. This arrangement is known as the periodic table. The periodic law ...
... electron's charge. The periodic law correlates very closely with the electronic structures of atoms. The law is demonstrated most clearly through a tabular arrangement that groups elements with similar properties together in an array. This arrangement is known as the periodic table. The periodic law ...
Honors Chemistry Unit 02
... quite), but atoms of different elements have different masses. – Atoms combine in simple, whole-number ratios to form compounds. – Atoms of one element cannot change into atoms of another element (not quite). In a chemical reaction, atoms change the way they are bound together with other atoms to fo ...
... quite), but atoms of different elements have different masses. – Atoms combine in simple, whole-number ratios to form compounds. – Atoms of one element cannot change into atoms of another element (not quite). In a chemical reaction, atoms change the way they are bound together with other atoms to fo ...
Nature of chemical reaction - Environmental-Chemistry
... Energy and chemical reactions: • Chemical reactions are breaking of old bonds from reactant-molecules and formation of new bonds in product-molecules. • Chemical reactions involve changes in energy. Photosynthesis is an endothermic reaction. • Energy is released (exothermic) during formation of bon ...
... Energy and chemical reactions: • Chemical reactions are breaking of old bonds from reactant-molecules and formation of new bonds in product-molecules. • Chemical reactions involve changes in energy. Photosynthesis is an endothermic reaction. • Energy is released (exothermic) during formation of bon ...
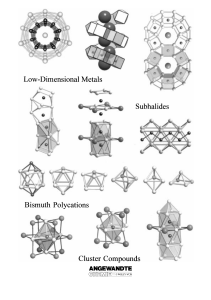
From the Metal to the Molecule
... 1) With the exception of cobalt, these elements form intermetallic phases with bismuth. Previous studies suggest that this is a basic requirement for the formation of the corresponding subhalides. Consequently, we can speak of the partial oxidation of intermetallic phases. 2) The electronegativities ...
... 1) With the exception of cobalt, these elements form intermetallic phases with bismuth. Previous studies suggest that this is a basic requirement for the formation of the corresponding subhalides. Consequently, we can speak of the partial oxidation of intermetallic phases. 2) The electronegativities ...
www.fahadsacademy.com
... Ionic bonding is the transfer of electrons from one atom to another to become achieve an inert gas configuration, forming ions. Ionic bonds are formed between METALLIC and NON- METALLIC ATOMS ONLY. - Metals lose electrons to form positive ions (cations) - Non-metals gain electrons to form negative i ...
... Ionic bonding is the transfer of electrons from one atom to another to become achieve an inert gas configuration, forming ions. Ionic bonds are formed between METALLIC and NON- METALLIC ATOMS ONLY. - Metals lose electrons to form positive ions (cations) - Non-metals gain electrons to form negative i ...
Name: Date: ______ 1. Which of the following is a property of both
... (1) A molecule is a group of two or more atoms that functions as a unit because the atoms are bound together by chemical forces. (2) The crushing of ice to make ice chips is a physical procedure that involves a chemical change. (3) Most naturally occurring samples of matter are mixtures rather than ...
... (1) A molecule is a group of two or more atoms that functions as a unit because the atoms are bound together by chemical forces. (2) The crushing of ice to make ice chips is a physical procedure that involves a chemical change. (3) Most naturally occurring samples of matter are mixtures rather than ...
Electron Configurations
... Along came Max Planck, who said that there is a fundamental restriction on the amounts of energy that an object emits or absorbs. He called these pieces of energy QUANTA. ...
... Along came Max Planck, who said that there is a fundamental restriction on the amounts of energy that an object emits or absorbs. He called these pieces of energy QUANTA. ...
Lecture 1 - Алтайский государственный технический
... 4. Compounds are formed when atoms of more than one element combine; a given compound always has the same relative number and kind of atoms. Atoms are the basic building blocks of matter; they are the smallest units of an element: an element is composed of only one kind of atom; in compounds the ato ...
... 4. Compounds are formed when atoms of more than one element combine; a given compound always has the same relative number and kind of atoms. Atoms are the basic building blocks of matter; they are the smallest units of an element: an element is composed of only one kind of atom; in compounds the ato ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.