Atom - Malibu High School
... and protons. Because the mass of the proton and the neutron is much larger than that of electrons, almost all the mass is located in the nucleus. Ion: a charged particle; # protons ≠ # electrons Electrons occupy most of the volume of an atom outside/around the nucleus. ...
... and protons. Because the mass of the proton and the neutron is much larger than that of electrons, almost all the mass is located in the nucleus. Ion: a charged particle; # protons ≠ # electrons Electrons occupy most of the volume of an atom outside/around the nucleus. ...
Problems - El Camino College
... c) The mass or an electron 1s about the same as the mass ofa proton. d) There are subatom ic particles in addition to the electron, proton. and neutron. e) The mass of an atom i~ uniformly distributed throughout the atom. f) Most of the particles fucd i11to the gold foi l in the Rutherford experimen ...
... c) The mass or an electron 1s about the same as the mass ofa proton. d) There are subatom ic particles in addition to the electron, proton. and neutron. e) The mass of an atom i~ uniformly distributed throughout the atom. f) Most of the particles fucd i11to the gold foi l in the Rutherford experimen ...
The Atomic Theory is Dead. Long Live the Atom
... 6th Century BC Kanada, founder of the Vaisheshika philosophy, held that the world was composed of atoms as many in kind as the various elements. Kanada believed light and heat to be varieties of the same substance; Udayana taught that all heat comes from the sun; and Vachaspati, like Newton, interpr ...
... 6th Century BC Kanada, founder of the Vaisheshika philosophy, held that the world was composed of atoms as many in kind as the various elements. Kanada believed light and heat to be varieties of the same substance; Udayana taught that all heat comes from the sun; and Vachaspati, like Newton, interpr ...
Triple Award - Cheltenham College
... sodium chloride, copper (II) sulphate and dilute sulfuric acid and predict the products. Write ionic half-‐equations representing the reactions at the electrodes during electrolysis. ...
... sodium chloride, copper (II) sulphate and dilute sulfuric acid and predict the products. Write ionic half-‐equations representing the reactions at the electrodes during electrolysis. ...
Worksheet 4 - Periodic Trends A number of physical and chemical
... However, not all electrons in an atom experience the same nuclear charge. Those closest to the nucleus experience the full nuclear charge and are held most strongly. As the number of electrons between the nucleus and the valence electrons increases, the apparent nuclear charge decreases, due to the ...
... However, not all electrons in an atom experience the same nuclear charge. Those closest to the nucleus experience the full nuclear charge and are held most strongly. As the number of electrons between the nucleus and the valence electrons increases, the apparent nuclear charge decreases, due to the ...
Chemistry 5.12 Spring 2003 Lectures #1 & 2, 2/5,7/03
... and molecular orbitals (σ, σ*, π, π*) Hybrid Atomic Orbital: Combination of atomic orbitals from the same ...
... and molecular orbitals (σ, σ*, π, π*) Hybrid Atomic Orbital: Combination of atomic orbitals from the same ...
chemistry intro and lesson 1
... second energy level and eight in the third energy level. We will not study the structure of atoms with more than three levels of energy levels. Each energy level must be filled before electrons occupy the next one. Electrons are so light they are considered to have zero mass. Electrons have a negati ...
... second energy level and eight in the third energy level. We will not study the structure of atoms with more than three levels of energy levels. Each energy level must be filled before electrons occupy the next one. Electrons are so light they are considered to have zero mass. Electrons have a negati ...
CHEM 101 Fall 09 Final Exam (a)
... 12. What is the frequency (s-1) of a photon that has an energy of 4.38 × 10-18 J? a. 436 b. 6.61 × 1015 c. 1.45 × 10-16 d. 2.30 × 107 e. 1.31 × 10-9 13. Which answer shows all possible values of the second quantum number when n = 3? a. l = 0 b. l = 0, 1 c. l = 0, 1, 2 d. l = 0, 1, 2, 3 e. l = 0, 1, ...
... 12. What is the frequency (s-1) of a photon that has an energy of 4.38 × 10-18 J? a. 436 b. 6.61 × 1015 c. 1.45 × 10-16 d. 2.30 × 107 e. 1.31 × 10-9 13. Which answer shows all possible values of the second quantum number when n = 3? a. l = 0 b. l = 0, 1 c. l = 0, 1, 2 d. l = 0, 1, 2, 3 e. l = 0, 1, ...
Principles of Technology
... a. Although Rutherford’s nuclear model was an important advance in our knowledge of atomic structure, it had two serious failings: Orbiting electrons are accelerating charges and, as such, should produce electromagnetic radiation. This release of energy should cause any electron’s orbit to decrease, ...
... a. Although Rutherford’s nuclear model was an important advance in our knowledge of atomic structure, it had two serious failings: Orbiting electrons are accelerating charges and, as such, should produce electromagnetic radiation. This release of energy should cause any electron’s orbit to decrease, ...
Hands-On Chemistry Unit
... 9. Reflection/Lessons Learned/Alterations for future use ........................................................................... 10. References and Resources.................................................................................................................. ...
... 9. Reflection/Lessons Learned/Alterations for future use ........................................................................... 10. References and Resources.................................................................................................................. ...
Honors Chemistry Worksheet – Atomic Theory and the Basic Atom
... of charged plates. The ions have the same charge, but the velocity of the germanium ions is twice as large as the velocity of the selenium ions. Identify which beam will be deflected more. Support your response. Remember; momentum is equal to mass times velocity (mv). The selenium - 74 ions would be ...
... of charged plates. The ions have the same charge, but the velocity of the germanium ions is twice as large as the velocity of the selenium ions. Identify which beam will be deflected more. Support your response. Remember; momentum is equal to mass times velocity (mv). The selenium - 74 ions would be ...
AP Chemistry: Bonding Multiple Choice
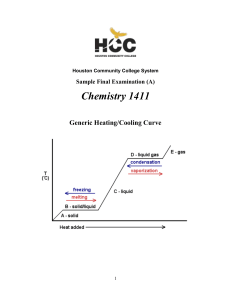
... (B) temperature at which the vapor pressure of the liquid is equal to 760 mm Hg. (C) temperature at which the solid, liquid, and vapor phases are all in equilibrium. (D) Temperature at which liquid and vapor phases are in equilibrium at I atmosphere. (E) lowest temperature above which a substance c ...
... (B) temperature at which the vapor pressure of the liquid is equal to 760 mm Hg. (C) temperature at which the solid, liquid, and vapor phases are all in equilibrium. (D) Temperature at which liquid and vapor phases are in equilibrium at I atmosphere. (E) lowest temperature above which a substance c ...
Isotopes and Atomic Mass
... • In college interviews, students wanted to select other common elements such as gold; investigation into other elements could be incorporated as part of an activity. • On the Mixtures screen, students attempted to match Nature’s Mix using My Mix view. This is not possible for all elements shown in ...
... • In college interviews, students wanted to select other common elements such as gold; investigation into other elements could be incorporated as part of an activity. • On the Mixtures screen, students attempted to match Nature’s Mix using My Mix view. This is not possible for all elements shown in ...
Nuclear physics is the subfield of physics that studies the building
... The discovery of artificial transmutation suggested that one approach would be was to either engage a neutron in a collision, or induce a collision that produced a neutron. Many experiments were tried. J. L. Glasson, a student at the Cavendish Laboratory, tried to aim high-velocity protons toward a ...
... The discovery of artificial transmutation suggested that one approach would be was to either engage a neutron in a collision, or induce a collision that produced a neutron. Many experiments were tried. J. L. Glasson, a student at the Cavendish Laboratory, tried to aim high-velocity protons toward a ...
Chapter 4 Notes
... Atomic Number – number of p+ in the nucleus of an atom (always equal to number of e-) Mass Number – number of p+ and n0 in the nucleus of an atom Atomic Weight (mass) – the average mass of the isotopes The mass number is just the atomic weight rounded off to a whole number!! Atomic Weight ...
... Atomic Number – number of p+ in the nucleus of an atom (always equal to number of e-) Mass Number – number of p+ and n0 in the nucleus of an atom Atomic Weight (mass) – the average mass of the isotopes The mass number is just the atomic weight rounded off to a whole number!! Atomic Weight ...
1411FINALSAMPLEs and Key
... sulfur atom in the first structure is therefore sp3. However, the sulfur is not simply sp3 hybridized in the second structure, which has an “expanded octet” around the sulfur atom. Hybridizations that allow more than an octet of electrons around an atom are sp3d (10 electrons) and sp3d2 (12 electron ...
... sulfur atom in the first structure is therefore sp3. However, the sulfur is not simply sp3 hybridized in the second structure, which has an “expanded octet” around the sulfur atom. Hybridizations that allow more than an octet of electrons around an atom are sp3d (10 electrons) and sp3d2 (12 electron ...
Document
... Mixtures – two or more substances that are not chemically combined with each other and can be separated by physical means. The substances in a mixture retain their individual properties. Solutions – a special kind of mixture where one substance dissolves in another. Elements – simplest form of pure ...
... Mixtures – two or more substances that are not chemically combined with each other and can be separated by physical means. The substances in a mixture retain their individual properties. Solutions – a special kind of mixture where one substance dissolves in another. Elements – simplest form of pure ...
Packet #7- Chemical Reactions
... to other atoms using chemical bonds. For example, iron and sulfur react together to form a compound called iron sulfide. Compounds usually have different properties from the elements they contain. Chemical formulas The chemical formula of a compound shows how many of each type of atom join together ...
... to other atoms using chemical bonds. For example, iron and sulfur react together to form a compound called iron sulfide. Compounds usually have different properties from the elements they contain. Chemical formulas The chemical formula of a compound shows how many of each type of atom join together ...
Carbene Singlets, Triplets, and the Physics that
... (molecular, in the likely event of two molecules interacting) orbitals, resulting in a mixing of pure orbitals. This mixing can either yield a bonding or an anti-bonding orbital, as seen in the interaction diagram (see figure 3). Orbital mixing diagrams like these are often qualitative, and while kn ...
... (molecular, in the likely event of two molecules interacting) orbitals, resulting in a mixing of pure orbitals. This mixing can either yield a bonding or an anti-bonding orbital, as seen in the interaction diagram (see figure 3). Orbital mixing diagrams like these are often qualitative, and while kn ...
Chapter One
... 0.1172 g of a pure hydrocarbon was burned in a C-H combustion train to produce 0.3509 g of CO2 and 0.1915 g of H2O. Determine the masses of C and H in the sample, the percentage of these elements in this hydrocarbon, and the empirical formula of the compound. ...
... 0.1172 g of a pure hydrocarbon was burned in a C-H combustion train to produce 0.3509 g of CO2 and 0.1915 g of H2O. Determine the masses of C and H in the sample, the percentage of these elements in this hydrocarbon, and the empirical formula of the compound. ...
IB Chemistry Online EQ_Ans
... chlorine (Cl2) and argon (Ar) are simple molecular covalent substances and hence are held together in the solid state by London (dispersion) forces. A small amount of thermal energy is required to break these intermolecular forces and hence their melting points are low. The strength or extent of Lon ...
... chlorine (Cl2) and argon (Ar) are simple molecular covalent substances and hence are held together in the solid state by London (dispersion) forces. A small amount of thermal energy is required to break these intermolecular forces and hence their melting points are low. The strength or extent of Lon ...
The Mole
... 0.1172 g of a pure hydrocarbon was burned in a C-H combustion train to produce 0.3509 g of CO2 and 0.1915 g of H2O. Determine the masses of C and H in the sample, the percentage of these elements in this hydrocarbon, and the empirical formula of the compound. ...
... 0.1172 g of a pure hydrocarbon was burned in a C-H combustion train to produce 0.3509 g of CO2 and 0.1915 g of H2O. Determine the masses of C and H in the sample, the percentage of these elements in this hydrocarbon, and the empirical formula of the compound. ...
Homework Booklet Unit 1 Feb14
... (c) Name the two pollutant gases changed by the catalyst and describe what they are changed into. 4. Explain why solid citric acid does not conduct electricity yet when it dissolves in water it does conduct. 5. Electrolysis of acids can be used to confirm the presence of hydrogen ions. (a) At which ...
... (c) Name the two pollutant gases changed by the catalyst and describe what they are changed into. 4. Explain why solid citric acid does not conduct electricity yet when it dissolves in water it does conduct. 5. Electrolysis of acids can be used to confirm the presence of hydrogen ions. (a) At which ...
National 5 Unit 1 Homework Booklet
... (c) Name the two pollutant gases changed by the catalyst and describe what they are changed into. 4. Explain why solid citric acid does not conduct electricity yet when it dissolves in water it does conduct. 5. Electrolysis of acids can be used to confirm the presence of hydrogen ions. (a) At which ...
... (c) Name the two pollutant gases changed by the catalyst and describe what they are changed into. 4. Explain why solid citric acid does not conduct electricity yet when it dissolves in water it does conduct. 5. Electrolysis of acids can be used to confirm the presence of hydrogen ions. (a) At which ...
Student Ch 11 Electrons-1
... amounts of energy • If we can’t fill these sublevels, then the next best thing is to be HALF full (one electron in each orbital in the sublevel) • There are many exceptions, but the most common ones are d4 and d9 For the purposes of this class, we are going to assume that ALL atoms (or ions) that en ...
... amounts of energy • If we can’t fill these sublevels, then the next best thing is to be HALF full (one electron in each orbital in the sublevel) • There are many exceptions, but the most common ones are d4 and d9 For the purposes of this class, we are going to assume that ALL atoms (or ions) that en ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.