Chemistry Unit Test Review
... Which is not a common physical property of Fe, Co, Ni, Cu, and Zn? ...
... Which is not a common physical property of Fe, Co, Ni, Cu, and Zn? ...
Monte Pettitt
... B. Montgomery Pettitt is the Hugh Roy and Lillie Cranz Cullen Professor of Chemistry and Professor of Physics, Computer Science, Biology and Biochemistry at The University of Houston. He earned his B.S. and Ph.D. from the University of Houston, was a Postdoctoral Fellow at the University of Texas at ...
... B. Montgomery Pettitt is the Hugh Roy and Lillie Cranz Cullen Professor of Chemistry and Professor of Physics, Computer Science, Biology and Biochemistry at The University of Houston. He earned his B.S. and Ph.D. from the University of Houston, was a Postdoctoral Fellow at the University of Texas at ...
This `practice exam`
... 19. What is the net ionic equation for the reaction of aqueous sodium carbonate with aqueous iron(III) chloride? 2 Fe3+(aq) + 3 CO32-(aq) → Fe2(CO3)3(s) 20. Dinitrogen trioxide, a blue solid, dissociates to form nitrogen monoxide and nitrogen dioxide gases. What mass of nitrogen dioxide is formed fr ...
... 19. What is the net ionic equation for the reaction of aqueous sodium carbonate with aqueous iron(III) chloride? 2 Fe3+(aq) + 3 CO32-(aq) → Fe2(CO3)3(s) 20. Dinitrogen trioxide, a blue solid, dissociates to form nitrogen monoxide and nitrogen dioxide gases. What mass of nitrogen dioxide is formed fr ...
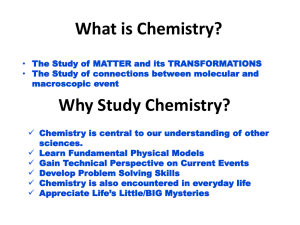
The Nature of Matter
... • Balances out protons positive charge • In constant motion • Valence electrons are in outermost shell • Valence electrons determine the chemical nature of an atom • Smallest subatomic particle ...
... • Balances out protons positive charge • In constant motion • Valence electrons are in outermost shell • Valence electrons determine the chemical nature of an atom • Smallest subatomic particle ...
Chemistry: The Basics
... – Discovered by James Chadwick in 1932. – Actual mass = 1.67 x 10-24 grams – No charge ...
... – Discovered by James Chadwick in 1932. – Actual mass = 1.67 x 10-24 grams – No charge ...
Chemistry - El Camino College
... and are called ______ or electrolytes 2. _________ Bonds are strong chemical bonds between atoms that result from the _______ of electrons in their outer orbitals. Molecules with covalent bonds are represented 2 ways: a. ___________ formulas in which each pair of shared electrons is represented by a ...
... and are called ______ or electrolytes 2. _________ Bonds are strong chemical bonds between atoms that result from the _______ of electrons in their outer orbitals. Molecules with covalent bonds are represented 2 ways: a. ___________ formulas in which each pair of shared electrons is represented by a ...
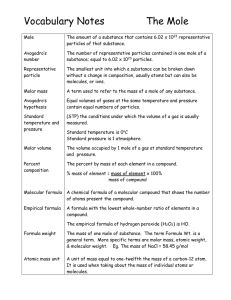
The Mole - TeacherWeb
... (This is actually how photoelectric cells create a current from cathode to anode.) Problem with photoelectric effect: this effect only occurs with light above a certain intensity. How come energy from low levels of light doesn't accumulate and eventually cause the photoelectric effect? Einstein expl ...
... (This is actually how photoelectric cells create a current from cathode to anode.) Problem with photoelectric effect: this effect only occurs with light above a certain intensity. How come energy from low levels of light doesn't accumulate and eventually cause the photoelectric effect? Einstein expl ...
PowerPoint Overview for Introduction
... whereas those appearing only at the level of parts per million or less are referred to as micronutrients. These nutrients perform various functions, including the building of bones and cell structures, regulating the body's pH, carrying charge, and driving chemical reactions. ...
... whereas those appearing only at the level of parts per million or less are referred to as micronutrients. These nutrients perform various functions, including the building of bones and cell structures, regulating the body's pH, carrying charge, and driving chemical reactions. ...
3rd Quarter Test
... 20) For a chemical system at equilibrium, a rise in temperature will a) favor the endothermic reaction b) favor the exothermic reaction c) decrease the rates of reaction d) have no effect upon the equilibrium 21) As 1 gram of H2O (g) changes to 1 gram of H2O (l), the entropy of the system a) decreas ...
... 20) For a chemical system at equilibrium, a rise in temperature will a) favor the endothermic reaction b) favor the exothermic reaction c) decrease the rates of reaction d) have no effect upon the equilibrium 21) As 1 gram of H2O (g) changes to 1 gram of H2O (l), the entropy of the system a) decreas ...
Chem 1A Midterm Exam Fall 2005
... 17. Extra: The following molecule is called phenylalanine. It is one of twenty common amino acids, all of which are building blocks for proteins. YOU (and any other living organism) are made of a myriad of proteins as well as many other kinds of biomolecules! Interestingly, our bodies cannot make p ...
... 17. Extra: The following molecule is called phenylalanine. It is one of twenty common amino acids, all of which are building blocks for proteins. YOU (and any other living organism) are made of a myriad of proteins as well as many other kinds of biomolecules! Interestingly, our bodies cannot make p ...
6.1 ATOMS, ELEMENTS, and COMPOUNDS
... by covalent bonds. • Can be a single, double, or triple bond depending on number of pairs of electrons shared. 2_____________________—forms when atom gives up electrons and another receives electrons in order to become stable • Electrical attraction between two oppositely charged atoms or groups of ...
... by covalent bonds. • Can be a single, double, or triple bond depending on number of pairs of electrons shared. 2_____________________—forms when atom gives up electrons and another receives electrons in order to become stable • Electrical attraction between two oppositely charged atoms or groups of ...
File - Ingolstadt Academy
... Matter, Change and Energy: Chemical and physical properties and changes Chemical symbols Ion formulas/charges Conservation of energy and mass Mixtures and Pure Substances Separation of Mixtures Scientific Measurement: Accuracy and Precision Significant Figures and Uncertainty Scien ...
... Matter, Change and Energy: Chemical and physical properties and changes Chemical symbols Ion formulas/charges Conservation of energy and mass Mixtures and Pure Substances Separation of Mixtures Scientific Measurement: Accuracy and Precision Significant Figures and Uncertainty Scien ...
Standard B-2
... o Enzymes are very specific. Each particular enzyme can catalyze only one chemical reaction by working on one particular reactant (substrate). o The structure of enzymes can be altered by temp and pH, so each catalyst works best at a specific temperature and pH. ...
... o Enzymes are very specific. Each particular enzyme can catalyze only one chemical reaction by working on one particular reactant (substrate). o The structure of enzymes can be altered by temp and pH, so each catalyst works best at a specific temperature and pH. ...
Vocabulary Notes
... without a change in composition, usually atoms but can also be molecules, or ions. ...
... without a change in composition, usually atoms but can also be molecules, or ions. ...
2. Chemistry of Living Things Outline
... catalyze. In organisms, _____________ allow the chemical reactions of ______________ to take place more efficiently than they otherwise would at body temperature. For example, amino acids are produced from protein digestion. The enzymes needed for this reaction are not changed but must be present fo ...
... catalyze. In organisms, _____________ allow the chemical reactions of ______________ to take place more efficiently than they otherwise would at body temperature. For example, amino acids are produced from protein digestion. The enzymes needed for this reaction are not changed but must be present fo ...
Chemistry of Living Things Outline
... reaction they catalyze. In organisms, _____________ allow the chemical reactions of ______________ to take place more efficiently than they otherwise would at body temperature. For example, amino acids are produced from protein digestion. The enzymes needed for this reaction are not changed but ...
... reaction they catalyze. In organisms, _____________ allow the chemical reactions of ______________ to take place more efficiently than they otherwise would at body temperature. For example, amino acids are produced from protein digestion. The enzymes needed for this reaction are not changed but ...
Activity 17 Follow-up
... very reactive. When the sodium reacts with the water it takes the place of one of the hydrogen atoms. This happens because sodium is more reactive than the hydrogen it is replacing. Reactivity is largely due to the atomic radius of an element and the valence. Larger metals lose their outer electrons ...
... very reactive. When the sodium reacts with the water it takes the place of one of the hydrogen atoms. This happens because sodium is more reactive than the hydrogen it is replacing. Reactivity is largely due to the atomic radius of an element and the valence. Larger metals lose their outer electrons ...
Section 2-4 “Chemical Reactions and Enzymes”
... Reactants – Elements or compounds that are the “starter materials” for a reaction Products – Elements or compounds produced by a chemical reaction ...
... Reactants – Elements or compounds that are the “starter materials” for a reaction Products – Elements or compounds produced by a chemical reaction ...