Introduction to Carbohydrates
... extracellular events & chemical changes within the cell - Many receptors signal their recognition of a bound ligand by initiating a series of reactions that ultimately result in a specific intracellular response - “second messenger” molecules, so named as they intervene b/w original messenger (neuro ...
... extracellular events & chemical changes within the cell - Many receptors signal their recognition of a bound ligand by initiating a series of reactions that ultimately result in a specific intracellular response - “second messenger” molecules, so named as they intervene b/w original messenger (neuro ...
Lab 6 Enzymes
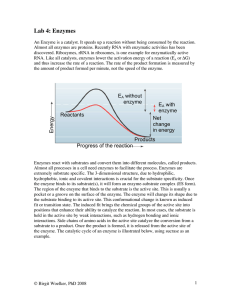
... Enzymes can be affected by other molecules. Many vitamins act as coenzymes, minerals act as cofactors for enzymes. Both will activate the enzymatic reaction by binding to the enzyme. Drugs and poisons are often inhibitors of enzymatic activity. Inhibitors may bind to the active site and directly com ...
... Enzymes can be affected by other molecules. Many vitamins act as coenzymes, minerals act as cofactors for enzymes. Both will activate the enzymatic reaction by binding to the enzyme. Drugs and poisons are often inhibitors of enzymatic activity. Inhibitors may bind to the active site and directly com ...
Novel physiological and metabolic insights into the beneficial
... fermented glucose into lactate, formate and butyrate as previously described for the batch fermentation of this bacterium in YCFAG medium 8. Figure 2a summarizes the observed bacterial growth (right panel) as well as the profiles for glucose consumption and the production of butyrate, formate and ac ...
... fermented glucose into lactate, formate and butyrate as previously described for the batch fermentation of this bacterium in YCFAG medium 8. Figure 2a summarizes the observed bacterial growth (right panel) as well as the profiles for glucose consumption and the production of butyrate, formate and ac ...
2 H+
... Chemiosmosis: The Energy-Coupling Mechanism § Electron transfer in the electron transport chain causes proteins to pump H+ from the mitochondrial matrix to the intermembrane space § H+ then moves back across the membrane, passing through the protein complex, ATP synthase § ATP synthase uses the ...
... Chemiosmosis: The Energy-Coupling Mechanism § Electron transfer in the electron transport chain causes proteins to pump H+ from the mitochondrial matrix to the intermembrane space § H+ then moves back across the membrane, passing through the protein complex, ATP synthase § ATP synthase uses the ...
Enzyme
... - The molecular structure corresponding to this maximum potential energy point is referred to as the “ transition state”—in the physical sense, the transition state can be envisioned as a transient pseudointermediate in the reaction with the maximum potential energy - Transition state (TS) theory po ...
... - The molecular structure corresponding to this maximum potential energy point is referred to as the “ transition state”—in the physical sense, the transition state can be envisioned as a transient pseudointermediate in the reaction with the maximum potential energy - Transition state (TS) theory po ...
Agoraphobia - Orthomolecular.org
... consumption are consistent with a defect in aerobic metabolism and a high anaerobic metabolism. (Cohen and White, p. 847). A characteristic of both disorders is breathlessness or air hunger which is particularly evident in crowded or stuffy atmospheres. Cohen and White noted that sufferers report av ...
... consumption are consistent with a defect in aerobic metabolism and a high anaerobic metabolism. (Cohen and White, p. 847). A characteristic of both disorders is breathlessness or air hunger which is particularly evident in crowded or stuffy atmospheres. Cohen and White noted that sufferers report av ...
Metabolomics - Circulation: Cardiovascular Genetics
... in hypoxia) and (2) to provide precursors for biosynthetic pathways. As traditionally envisaged (eg, in most biochemistry textbooks), the CAC begins with the condensation of oxaloacetate with acetyl-CoA, consisting of 8 sequential linear irreversible (eg, citrate synthase and the 2-oxoglutarate dehy ...
... in hypoxia) and (2) to provide precursors for biosynthetic pathways. As traditionally envisaged (eg, in most biochemistry textbooks), the CAC begins with the condensation of oxaloacetate with acetyl-CoA, consisting of 8 sequential linear irreversible (eg, citrate synthase and the 2-oxoglutarate dehy ...
Lecture 27
... ruminants and the human colon. • b-oxidation occurs pretty much as w/ even chain fatty acids until the final thiolase cleavage which results in a 3 carbon acyl-CoA (propionyl-CoA) • Special set of 3 enzymes are required to further oxidize propionyl-CoA • Final Product succinyl-CoA enters TCA cycle ...
... ruminants and the human colon. • b-oxidation occurs pretty much as w/ even chain fatty acids until the final thiolase cleavage which results in a 3 carbon acyl-CoA (propionyl-CoA) • Special set of 3 enzymes are required to further oxidize propionyl-CoA • Final Product succinyl-CoA enters TCA cycle ...
AULAS DE BIOQUÍMICA
... The dimeric functional unit. Cytochrome c1 and the Rieske iron-sulfur protein project from the P surface and can interact with cytochrome c in the intermembrane space. The complex has two distinct binding sites for ubiquinone, QN and QP, The Q cycle: On the P side of the membrane, two molecules of Q ...
... The dimeric functional unit. Cytochrome c1 and the Rieske iron-sulfur protein project from the P surface and can interact with cytochrome c in the intermembrane space. The complex has two distinct binding sites for ubiquinone, QN and QP, The Q cycle: On the P side of the membrane, two molecules of Q ...
Chapter 8 – an introduction to metabolism
... 8. Describe how the carbon skeleton of glucose changes as it proceeds through glycolysis. 9. Explain why ATP is required for the preparatory steps of glycolysis. 10. Identify where substrate-level phosphorylation and the reduction of NAD+ occur in glycolysis. 11. Describe where pyruvate is oxidized ...
... 8. Describe how the carbon skeleton of glucose changes as it proceeds through glycolysis. 9. Explain why ATP is required for the preparatory steps of glycolysis. 10. Identify where substrate-level phosphorylation and the reduction of NAD+ occur in glycolysis. 11. Describe where pyruvate is oxidized ...
... courses of pNP formation from reactions of 21B3 on 12pNCA in 1 mm, 5 mm, and 10 mm H2O2. The enzyme is essentially inactive within 5 minutes in 10 mm H2O2. Inactivation appears to be turnover-dependent (similar TONs are reached from reactions in 1 mm, 5 mm, and 10 mm H2O2), and the ratio of peroxyge ...
Principles of BIOCHEMISTRY
... • Table 7.1 Vitamins, nutritional deficiency diseases, p194 Prentice Hall c2002 ...
... • Table 7.1 Vitamins, nutritional deficiency diseases, p194 Prentice Hall c2002 ...
Cellular Respiration - Chandler Unified School District
... glucose followed by lactic acid production. • As the cells do not own any protein coding DNA they cannot produce new structural or repair proteins or enzymes and their lifespan is limited. ...
... glucose followed by lactic acid production. • As the cells do not own any protein coding DNA they cannot produce new structural or repair proteins or enzymes and their lifespan is limited. ...
Acyl-CoA
... - Triglycerides (or triacylglycerols) are fatty acid esters (usually with different fatty acid R groups) of glycerol—see §1.4! - Triglycerides are largely stored in the adipose tissue where they function as “high-energy” reservoirs—due to being more reduced (carry more electrons, or more hydrogens!) ...
... - Triglycerides (or triacylglycerols) are fatty acid esters (usually with different fatty acid R groups) of glycerol—see §1.4! - Triglycerides are largely stored in the adipose tissue where they function as “high-energy” reservoirs—due to being more reduced (carry more electrons, or more hydrogens!) ...
Study Guide A - The Science of Payne
... 1. Cellular respiration is a process that releases glucose / energy from sugars and other carbon-based molecules to make ATP when oxygen / carbon dioxide is present. 2. Cellular respiration is called an aerobic process, because it needs oxygen / carbon dioxide to take place. 3. Cellular respiration ...
... 1. Cellular respiration is a process that releases glucose / energy from sugars and other carbon-based molecules to make ATP when oxygen / carbon dioxide is present. 2. Cellular respiration is called an aerobic process, because it needs oxygen / carbon dioxide to take place. 3. Cellular respiration ...
Roberts, LM Dept. of Chemistry California State
... Be able to predict regulation of the TCA cycle Understand the basic concepts of the glyoxylate cycle (two unique reactions, 4 carbons feed in as AcCoA, 4 carbons come out as succinate; these carbons are precursors for carbohydrates. Plants can make CHO from fatty acid carbons but animals cannot). Kn ...
... Be able to predict regulation of the TCA cycle Understand the basic concepts of the glyoxylate cycle (two unique reactions, 4 carbons feed in as AcCoA, 4 carbons come out as succinate; these carbons are precursors for carbohydrates. Plants can make CHO from fatty acid carbons but animals cannot). Kn ...
REAL Health Solutions!
... word ─ LIFE. Without systemic enzymes, LIFE itself is not possible, not for people, plants, or animals.** Enzymes are the single most important essential for every reaction taking place in a living organism because every second of everyday life changes and renews itself. Restoring proper levels of s ...
... word ─ LIFE. Without systemic enzymes, LIFE itself is not possible, not for people, plants, or animals.** Enzymes are the single most important essential for every reaction taking place in a living organism because every second of everyday life changes and renews itself. Restoring proper levels of s ...
T06 Fermentations 2014
... • NAHD accumulates and NAD+ is depleted • TCA cycle (requiring NAD+) can’t run • glucose uptake stops NADH (or NADPH) can also be used for anabolism (assimilation) but in addition to reducing power also ATP is needed for assimilation ...
... • NAHD accumulates and NAD+ is depleted • TCA cycle (requiring NAD+) can’t run • glucose uptake stops NADH (or NADPH) can also be used for anabolism (assimilation) but in addition to reducing power also ATP is needed for assimilation ...
How to ID an Unknown Organism
... IMViC test, page 6). You could also use a tube of SIM media. Use a needle to obtain the inoculum. Stab the needle into the motility medium, almost all the way to the bottom, then pull the needle back out in a straight line, backing the needle out of the same stab line you made going in. Remember, th ...
... IMViC test, page 6). You could also use a tube of SIM media. Use a needle to obtain the inoculum. Stab the needle into the motility medium, almost all the way to the bottom, then pull the needle back out in a straight line, backing the needle out of the same stab line you made going in. Remember, th ...
28 - Weebly
... • Proteins are important structural and functional molecules in the body. • The amino acids from proteins may be used for synthesis of new molecules, or may be burned for energy. • Healthy rates of protein synthesis require a homeostatically regulated nitrogen balance, which compares the rate of inc ...
... • Proteins are important structural and functional molecules in the body. • The amino acids from proteins may be used for synthesis of new molecules, or may be burned for energy. • Healthy rates of protein synthesis require a homeostatically regulated nitrogen balance, which compares the rate of inc ...
8 - student.ahc.umn.edu
... cytochrome c1 to cytochrome c. The other electron goes to a protein bound coenzyme Q molecule to form a free radical, then through two variants of cytochrome b and finally ends up in a second coenzyme Q as a free radical with an unpaired electron. This free radical intermediate is extremely reactiv ...
... cytochrome c1 to cytochrome c. The other electron goes to a protein bound coenzyme Q molecule to form a free radical, then through two variants of cytochrome b and finally ends up in a second coenzyme Q as a free radical with an unpaired electron. This free radical intermediate is extremely reactiv ...
Module 6 – Microbial Metabolism
... avoid the overproduction of NADH, obligately fermentative organisms usually do not have a complete citric acid cycle. Instead of using an ATP synthase as in respiration, ATP in fermentative organisms is produced by substrate-level phosphorylation where a phosphate group is transferred from a high-en ...
... avoid the overproduction of NADH, obligately fermentative organisms usually do not have a complete citric acid cycle. Instead of using an ATP synthase as in respiration, ATP in fermentative organisms is produced by substrate-level phosphorylation where a phosphate group is transferred from a high-en ...
fulltext
... to sugar alcohols and cyclitols includes the biosynthetic pathways leading to glucitol, inositols, and pseudosaccharides. Extensively used are reaction schemes, sequences, and mechanisms with the enzymes involved and detailed explanations employing sound chemical principles and mechanisms. Keywords: ...
... to sugar alcohols and cyclitols includes the biosynthetic pathways leading to glucitol, inositols, and pseudosaccharides. Extensively used are reaction schemes, sequences, and mechanisms with the enzymes involved and detailed explanations employing sound chemical principles and mechanisms. Keywords: ...
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery.In organisms, NAD can be synthesized from simple building-blocks (de novo) from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.Although NAD+ is written with a superscript plus sign because of the formal charge on a particular nitrogen atom, at physiological pH for the most part it is actually a singly charged anion (charge of minus 1), while NADH is a doubly charged anion.