... cerevisiae such that it can reoxidize NADH by the reduction of acetic acid to ethanol via NADH-dependent reactions. Acetic acid is available at significant amounts in lignocellulosic hydrolysates of agricultural residues. Consistent with earlier studies, deletion of the two genes encoding NAD-depend ...
Factors affecting enzyme activity ppt - Mr. Lesiuk
... Catalysts are substances that speed up chemical reactions. Organic catalysts (contain carbon) are called enzymes. Enzymes are specific for one particular reaction or group of related reactions. Many reactions cannot occur without the correct enzyme present. They are often named by adding “ASE" to th ...
... Catalysts are substances that speed up chemical reactions. Organic catalysts (contain carbon) are called enzymes. Enzymes are specific for one particular reaction or group of related reactions. Many reactions cannot occur without the correct enzyme present. They are often named by adding “ASE" to th ...
Document
... Catalysts are substances that speed up chemical reactions. Organic catalysts (contain carbon) are called enzymes. Enzymes are specific for one particular reaction or group of related reactions. Many reactions cannot occur without the correct enzyme present. They are often named by adding “ASE" to th ...
... Catalysts are substances that speed up chemical reactions. Organic catalysts (contain carbon) are called enzymes. Enzymes are specific for one particular reaction or group of related reactions. Many reactions cannot occur without the correct enzyme present. They are often named by adding “ASE" to th ...
Unit 4 Notes - heckgrammar.co.uk
... 5. GP is converted in a series of steps to form the 3-carbon compound pyruvate. Another ATP is made during this process. Pyruvate marks the end of glycolysis, the first stage of respiration. Pyruvate can also be turned back into glucose by reversing glycolysis, and this is called gluconeogenesis. 6. ...
... 5. GP is converted in a series of steps to form the 3-carbon compound pyruvate. Another ATP is made during this process. Pyruvate marks the end of glycolysis, the first stage of respiration. Pyruvate can also be turned back into glucose by reversing glycolysis, and this is called gluconeogenesis. 6. ...
The Tricarboxylic Acid Cycle in Thiobacillus
... Results are further discussed in relation to possible reasons for autotrophy. INTRODUCTION ...
... Results are further discussed in relation to possible reasons for autotrophy. INTRODUCTION ...
Principles of BIOCHEMISTRY - Illinois State University
... a-ketoglutarate (a metabolically irreversible reaction) ...
... a-ketoglutarate (a metabolically irreversible reaction) ...
Enzyme Optimum pH - Sir Sabir Hussain
... An enzyme and its substrate react with each through a specific charge bearing site of an enzyme called active site The charge and shape of the active site is formed by some amino acids present in the polypeptide chain of the active site of the enzyme These amino acids are brought closer and are arra ...
... An enzyme and its substrate react with each through a specific charge bearing site of an enzyme called active site The charge and shape of the active site is formed by some amino acids present in the polypeptide chain of the active site of the enzyme These amino acids are brought closer and are arra ...
Document
... •Glyceraldehyde-3-PO4 is both an intermediate and final product •Fructose-6-PO4 is never used as an intermediate, return to the glycolytic pathway ...
... •Glyceraldehyde-3-PO4 is both an intermediate and final product •Fructose-6-PO4 is never used as an intermediate, return to the glycolytic pathway ...
Embden-Meyerhof-Parnas Pathway
... • It can store glucose as glycogen and release glucose into the blood stream when [glucose] falls. ...
... • It can store glucose as glycogen and release glucose into the blood stream when [glucose] falls. ...
active site - Blue Valley Schools
... Substrates held in active site by weak interactions, such as hydrogen bonds and ionic bonds. ...
... Substrates held in active site by weak interactions, such as hydrogen bonds and ionic bonds. ...
(β/α)8-barrel enzymes present in completely sequenced genomes
... focused on the three rest (β/α)8 -barrels. The genes encoding the triosephosphate isomerase, enolase and pyruvate kinase were found in 43 genomes. The corresponding amino acid sequences were collected and aligned, and the three respective evolutionary trees were constructed and discussed. The result ...
... focused on the three rest (β/α)8 -barrels. The genes encoding the triosephosphate isomerase, enolase and pyruvate kinase were found in 43 genomes. The corresponding amino acid sequences were collected and aligned, and the three respective evolutionary trees were constructed and discussed. The result ...
Citric acid cycle - Imperial College London
... converted into acetyl-CoA by decarboxylation and enters the citric acid cycle. In protein catabolism, proteins are broken down by proteases into their constituent amino acids. The carbon backbone of these amino acids can become a source of energy by being converted to acetyl-CoA and entering into th ...
... converted into acetyl-CoA by decarboxylation and enters the citric acid cycle. In protein catabolism, proteins are broken down by proteases into their constituent amino acids. The carbon backbone of these amino acids can become a source of energy by being converted to acetyl-CoA and entering into th ...
Recent developments in photorespiration research
... enediolate, O2 can replace CO2 as a substrate, forming 3PGA and 2PG (2-phosphoglycolate). In contrast with 3PGA, 2PG cannot directly enter the Calvin cycle, and its accumulation is toxic. Rubisco strongly favours CO2 over O2 , but cellular CO2 concentration is typically low and oxygen is produced in ...
... enediolate, O2 can replace CO2 as a substrate, forming 3PGA and 2PG (2-phosphoglycolate). In contrast with 3PGA, 2PG cannot directly enter the Calvin cycle, and its accumulation is toxic. Rubisco strongly favours CO2 over O2 , but cellular CO2 concentration is typically low and oxygen is produced in ...
Biochemistry Study Guide NITROGEN METABOLISM
... 2 ATP are required. Basically these are used to "charge" or "activate" ammonia with a highenergy phosphate bond, before we subsequently start urea synthesis. N-Acetylglutamate is absolutely required as a cofactor. This compound also serves a regulatory role in urea synthesis. The rate of carba ...
... 2 ATP are required. Basically these are used to "charge" or "activate" ammonia with a highenergy phosphate bond, before we subsequently start urea synthesis. N-Acetylglutamate is absolutely required as a cofactor. This compound also serves a regulatory role in urea synthesis. The rate of carba ...
BCMB 3100 – Chapters 6,7,8 Enzyme Basics • Six Classes (IUBMB
... k2 / Km is limited by k1 which is limited < 109M-1 s-1 (due to limits of diffusion). A few enzymes catalyze reactions at this upper physical rate = diffusion controlled reactions. ...
... k2 / Km is limited by k1 which is limited < 109M-1 s-1 (due to limits of diffusion). A few enzymes catalyze reactions at this upper physical rate = diffusion controlled reactions. ...
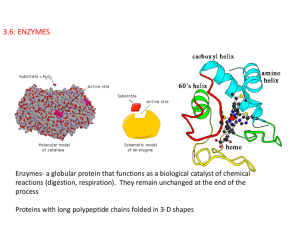
3.6: ENZYMES
... contain many +/- regions, some around the active site. An excess of H+ ions in an acidic solution can lead to bonding between the H+ ions and the negative charges in the active site. (same with OH- in basic solutions where the OH- ions bond to the positive sites). This will inhibit the matching proc ...
... contain many +/- regions, some around the active site. An excess of H+ ions in an acidic solution can lead to bonding between the H+ ions and the negative charges in the active site. (same with OH- in basic solutions where the OH- ions bond to the positive sites). This will inhibit the matching proc ...
Uria_et_al_2006 _ADH paper
... In recent years, enzymes belonging to the oxidoreductase group such as alcohol dehydrogenases (ADHs) have caught much scientific attention in the pharmaceutical industries. They are highly desirable as biological catalysts in the production of chiral pharmaceutical intermediates or building blocks i ...
... In recent years, enzymes belonging to the oxidoreductase group such as alcohol dehydrogenases (ADHs) have caught much scientific attention in the pharmaceutical industries. They are highly desirable as biological catalysts in the production of chiral pharmaceutical intermediates or building blocks i ...
Enzymes - JLooby Biology
... If a source of enzyme is placed in the agar plate, the enzyme will diffuse out through the agar, turning the substrate into product as it goes. There must be a way to distinguish the substrate from the product, and the reaction will then show up as a ring around the enzyme source. The higher the con ...
... If a source of enzyme is placed in the agar plate, the enzyme will diffuse out through the agar, turning the substrate into product as it goes. There must be a way to distinguish the substrate from the product, and the reaction will then show up as a ring around the enzyme source. The higher the con ...
Document
... 7.1.2 Charged intermediates can often be stabilized by transferring protons to or from the substrate or intermediate to form a species that breaks down to products more readily than to reactants. Catalysis here means the facilitated (coordinated, aligned) proton transfer. 7.1.3 General acid-base ca ...
... 7.1.2 Charged intermediates can often be stabilized by transferring protons to or from the substrate or intermediate to form a species that breaks down to products more readily than to reactants. Catalysis here means the facilitated (coordinated, aligned) proton transfer. 7.1.3 General acid-base ca ...
Lab 5 Sugar Fermentation in Yeast
... Anaerobic energy production in yeast will be studied in this lab investigation. In this lab you will determine which sugar, sucrose or lactose, is best metabolized by yeast, while in Part II you will design an experiment to determine the effect ethanol has on the rate of fermentation. Cultures aroun ...
... Anaerobic energy production in yeast will be studied in this lab investigation. In this lab you will determine which sugar, sucrose or lactose, is best metabolized by yeast, while in Part II you will design an experiment to determine the effect ethanol has on the rate of fermentation. Cultures aroun ...
Biocatalytic degradation of pollutants
... a member of the major facilitator superfamily of transport proteins. These reports are of interest, because they indicate that these bacteria have not only evolved new pathways for the degradation of man-made chemicals, but that they have also evolved appropriate chemosensory systems for their detec ...
... a member of the major facilitator superfamily of transport proteins. These reports are of interest, because they indicate that these bacteria have not only evolved new pathways for the degradation of man-made chemicals, but that they have also evolved appropriate chemosensory systems for their detec ...
Cellular Respiration
... receives a phosphate group. The product of this step is two molecules of a new three-carbon compound. As shown in Figure 7-3, the oxidation of G3P is accompanied by the reduction of two molecules of nicotinamide adenine dinucleotide (NAD!) to NADH. NAD! is similar to NADP!, a compound involved in th ...
... receives a phosphate group. The product of this step is two molecules of a new three-carbon compound. As shown in Figure 7-3, the oxidation of G3P is accompanied by the reduction of two molecules of nicotinamide adenine dinucleotide (NAD!) to NADH. NAD! is similar to NADP!, a compound involved in th ...
Mary Jones Jennifer Gregory - Assets
... In the past, the bonds attaching the two outer phosphate groups have been called ‘high-energy bonds’, because more energy is released when they are broken than when the last phosphate is removed. This is misleading and should be The structure of adenosine triphosphate (ATP) is avoided since the ener ...
... In the past, the bonds attaching the two outer phosphate groups have been called ‘high-energy bonds’, because more energy is released when they are broken than when the last phosphate is removed. This is misleading and should be The structure of adenosine triphosphate (ATP) is avoided since the ener ...
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery.In organisms, NAD can be synthesized from simple building-blocks (de novo) from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.Although NAD+ is written with a superscript plus sign because of the formal charge on a particular nitrogen atom, at physiological pH for the most part it is actually a singly charged anion (charge of minus 1), while NADH is a doubly charged anion.