You Light Up My Life
... • Coenzymes give up electrons to electron transport system • Electrons are transported through the system • The final electron acceptor is oxygen • H+ is moved from inner to outer compartment ...
... • Coenzymes give up electrons to electron transport system • Electrons are transported through the system • The final electron acceptor is oxygen • H+ is moved from inner to outer compartment ...
L3 - Bacterial Metabolism v4
... • Photosynthetic organisms obtain energy from… • Chemoorganotrophs obtain energy from….. ...
... • Photosynthetic organisms obtain energy from… • Chemoorganotrophs obtain energy from….. ...
Cellular Respiration NOTES
... mitochondria. As the electrons move through the chain they release lots of energy to pump H+ ions into the intermembrane space. In doing so, a steep concentration gradient is formed and H+ ions will flow back into the matrix through the membrane protein ATP synthase. In flowing back through the H+ p ...
... mitochondria. As the electrons move through the chain they release lots of energy to pump H+ ions into the intermembrane space. In doing so, a steep concentration gradient is formed and H+ ions will flow back into the matrix through the membrane protein ATP synthase. In flowing back through the H+ p ...
Introduction to: Cellular Respiration
... Like a bank, you put money in to earn interest. Net ATP gained per glucose molecule=2 ...
... Like a bank, you put money in to earn interest. Net ATP gained per glucose molecule=2 ...
Six Major Classes of Enzymes and Examples of Their Subclasses
... positively charged pyridine ring is a strong electron-withdrawing group which pulls electrons into it from the bonds around the amino acid αcarbon (electrophilic catalysis). NAD+ accepts a hydride ion, shown in blue. NAD+-dependent dehydrogenases catalyze the transfer of a hydride ion (H:-) from a c ...
... positively charged pyridine ring is a strong electron-withdrawing group which pulls electrons into it from the bonds around the amino acid αcarbon (electrophilic catalysis). NAD+ accepts a hydride ion, shown in blue. NAD+-dependent dehydrogenases catalyze the transfer of a hydride ion (H:-) from a c ...
SOME Important Points About Cellular Energetics by Dr. Ty C.M.
... In the absence of oxygen (or in cells that do not have mitochondria), glycolysis is the only process available to produce ATP from the energy in glucose. This is called anaerobic metabolism. If glyc ...
... In the absence of oxygen (or in cells that do not have mitochondria), glycolysis is the only process available to produce ATP from the energy in glucose. This is called anaerobic metabolism. If glyc ...
Camp 1
... • CoA is often written CoA-SH to emphasize the fact that it contains a sulfhydryl group. • The vitamin part of coenzyme A is pantothenic acid. • The acetyl group of acetyl CoA is bound as a highenergy thioester. O CH3 -C-S-CoA Acetyl coenzyme A (An acyl CoA) ...
... • CoA is often written CoA-SH to emphasize the fact that it contains a sulfhydryl group. • The vitamin part of coenzyme A is pantothenic acid. • The acetyl group of acetyl CoA is bound as a highenergy thioester. O CH3 -C-S-CoA Acetyl coenzyme A (An acyl CoA) ...
Midterm Exam Advanced Biochemistry II (Answer) 1. At equilibrium
... muscle tissue is vastly increased. In rabbit leg muscle or turkey flight muscle, the ATP is produced almost exclusively by lactic acid fermentation. ATP is formed in the payoff phase of glycolysis by two reactions, promoted by phosphoglycerate kinase and pyruvate kinase. Suppose skeletal muscle were ...
... muscle tissue is vastly increased. In rabbit leg muscle or turkey flight muscle, the ATP is produced almost exclusively by lactic acid fermentation. ATP is formed in the payoff phase of glycolysis by two reactions, promoted by phosphoglycerate kinase and pyruvate kinase. Suppose skeletal muscle were ...
Practice Test - IHS AP Biology
... B) the oxidation of glucose and other organic compounds. C) the H+ concentration gradient across the inner mitochondrial membrane. D) the affinity of oxygen for electrons. E) the transfer of phosphate to ADP. ...
... B) the oxidation of glucose and other organic compounds. C) the H+ concentration gradient across the inner mitochondrial membrane. D) the affinity of oxygen for electrons. E) the transfer of phosphate to ADP. ...
File
... • Light is a form of electromagnetic radiation, which travels as a wave but also behaves as particles (photons). • Photons can be absorbed by a molecule, adding energy to the molecule—it moves to an excited state. ...
... • Light is a form of electromagnetic radiation, which travels as a wave but also behaves as particles (photons). • Photons can be absorbed by a molecule, adding energy to the molecule—it moves to an excited state. ...
The Kreb`s Cycle - hrsbstaff.ednet.ns.ca
... The cristae allow for a greater surface area for chemical reactions to occur. This is the part of cellular respiration that oxygen is used. • Chemiosmosis – The process in which energy stored in the form of a hydrogen ion gradient across a membrane is used to drive cellular work, such as the synthes ...
... The cristae allow for a greater surface area for chemical reactions to occur. This is the part of cellular respiration that oxygen is used. • Chemiosmosis – The process in which energy stored in the form of a hydrogen ion gradient across a membrane is used to drive cellular work, such as the synthes ...
Document
... The ETC couples the transfer of electrons between a donor (like NADH) and an electron acceptor (like O2) with the transfer of protons (H+ ions) across the inner mitochondrial membrane, enabling the process of oxidative phosphorylation. In the presence of oxygen, energy is passed, stepwise, through t ...
... The ETC couples the transfer of electrons between a donor (like NADH) and an electron acceptor (like O2) with the transfer of protons (H+ ions) across the inner mitochondrial membrane, enabling the process of oxidative phosphorylation. In the presence of oxygen, energy is passed, stepwise, through t ...
Structure and function of mitochondria (Slide
... Controlled decomposition of pyruvate Releases carbon as CO2 H+ ions captured by NAD Releases 2 ATP Provides > 20 proteins for metabolic processes Refer to p127 in Biozone Look at position on flowchart ...
... Controlled decomposition of pyruvate Releases carbon as CO2 H+ ions captured by NAD Releases 2 ATP Provides > 20 proteins for metabolic processes Refer to p127 in Biozone Look at position on flowchart ...
word
... Role of CoASH and of carnitine; describe cycles of -oxidation, need for ATP at beginning, what are products at end from typical LCFA? Role of oxygen? Fatty acids used as fuels and when, which are most common FA? Acyl CoA synthetases specificity for chain length FA Energy yield from fatty acid oxida ...
... Role of CoASH and of carnitine; describe cycles of -oxidation, need for ATP at beginning, what are products at end from typical LCFA? Role of oxygen? Fatty acids used as fuels and when, which are most common FA? Acyl CoA synthetases specificity for chain length FA Energy yield from fatty acid oxida ...
Cellular respiration
... Partial oxidation of glucose to form pyruvic acid. A small amount of ATP is made. Some NAD is reduced to form NADH. The major glycolytic pathway in cells is the ...
... Partial oxidation of glucose to form pyruvic acid. A small amount of ATP is made. Some NAD is reduced to form NADH. The major glycolytic pathway in cells is the ...
Matabolic Stoichiometry and Energetics in
... reducing power made available during breakdown of nutrient is carried to biosynthetic reaction. The reducing power is used for the construction of cell components. ...
... reducing power made available during breakdown of nutrient is carried to biosynthetic reaction. The reducing power is used for the construction of cell components. ...
Iron-sulfur proteins
... • When cell receives a signal for apoptosis, one consequence is the permeability of the outer mitochondrial membrane will increase, allowing cytochrome c release. • The release of cytochrome c will activate caspase 9, which will initiate the protein ...
... • When cell receives a signal for apoptosis, one consequence is the permeability of the outer mitochondrial membrane will increase, allowing cytochrome c release. • The release of cytochrome c will activate caspase 9, which will initiate the protein ...
Jeopardy Review Enzyme/Energetics
... The process of breaking down pyruvates in the absence of oxygen to obtain energy ...
... The process of breaking down pyruvates in the absence of oxygen to obtain energy ...
5 Metabolism - bloodhounds Incorporated
... The final electron acceptor in the process of oxidative phosphorylation is A. B. C. D. ...
... The final electron acceptor in the process of oxidative phosphorylation is A. B. C. D. ...
Glycolysis is the first stage of cellular respiration
... group of enzymes located in the cistae of the mitochondria, they are converted to Acetyl ACO. During the conversion of pyruvate to Aceytl ACO a CO2 and FADH2 molecule are produced. The Krebs cycle the second stage of cellular respiration: a series of reactions catalyzed by enzymes located in the mit ...
... group of enzymes located in the cistae of the mitochondria, they are converted to Acetyl ACO. During the conversion of pyruvate to Aceytl ACO a CO2 and FADH2 molecule are produced. The Krebs cycle the second stage of cellular respiration: a series of reactions catalyzed by enzymes located in the mit ...
Fact Sheet - Advanced Equine Solutions
... reactions used by all aerobic organisms to generate energy through the oxidation of acetate derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in the form of adenosine triphosphate(ATP). In addition, the cycle provides precursors of certain amino acids as well as t ...
... reactions used by all aerobic organisms to generate energy through the oxidation of acetate derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in the form of adenosine triphosphate(ATP). In addition, the cycle provides precursors of certain amino acids as well as t ...
Anaerobic Fermentation
... Lasts only 46 seconds. 2. Lactic Acid Fermentation Lasts up to 90 seconds (sprints) ...
... Lasts only 46 seconds. 2. Lactic Acid Fermentation Lasts up to 90 seconds (sprints) ...
Cellular Metabolism - Oklahoma State University–Stillwater
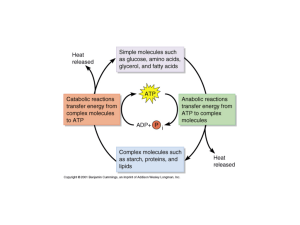
... • Large molecules are broken into smaller molecules (catabolism) • Critical intermediates are generated (ex. Pyruvic acid, etc.) They are used in the anabolism process as well as entry back into the catabolism process. ...
... • Large molecules are broken into smaller molecules (catabolism) • Critical intermediates are generated (ex. Pyruvic acid, etc.) They are used in the anabolism process as well as entry back into the catabolism process. ...
Enzyme Fundamental Concepts Enzymes are biological catalysts
... 3. Enzymes speed up chemical reactions by lowering activation energy. 4. Enzymes catalyze exergonic (exothermic)/ spontaneous reactions. 5. Enzymes are unused and unchanged by the chemical reaction they catalyze. 6. Enzymes catalyze one specific chemical reaction. 7. An increase in the concentration ...
... 3. Enzymes speed up chemical reactions by lowering activation energy. 4. Enzymes catalyze exergonic (exothermic)/ spontaneous reactions. 5. Enzymes are unused and unchanged by the chemical reaction they catalyze. 6. Enzymes catalyze one specific chemical reaction. 7. An increase in the concentration ...
Lecture #4 Date
... • Krebs Cycle: location mitochondrial matrix • Electron Transport Chain location: inner membrane of ...
... • Krebs Cycle: location mitochondrial matrix • Electron Transport Chain location: inner membrane of ...
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery.In organisms, NAD can be synthesized from simple building-blocks (de novo) from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.Although NAD+ is written with a superscript plus sign because of the formal charge on a particular nitrogen atom, at physiological pH for the most part it is actually a singly charged anion (charge of minus 1), while NADH is a doubly charged anion.