Physics 272: Electricity and Magnetism
... Structure of atoms • Atoms are made up of protons, neutrons, and electrons • The nucleus is a tiny object at the center of the atom • An electron cloud surrounds the nucleus • Question: If the nucleus is made up of protons and neutrons, which are Larger than electrons, why is the electron cloud so ...
... Structure of atoms • Atoms are made up of protons, neutrons, and electrons • The nucleus is a tiny object at the center of the atom • An electron cloud surrounds the nucleus • Question: If the nucleus is made up of protons and neutrons, which are Larger than electrons, why is the electron cloud so ...
Unit 01 Qual Chem
... Pure Substances – Only one type of matter is present Mixtures- More than one type of matter is present but each kind retains its own properties. Can be separated by physical means ...
... Pure Substances – Only one type of matter is present Mixtures- More than one type of matter is present but each kind retains its own properties. Can be separated by physical means ...
On the energy of electric field in hydrogen atom
... which is present in the atom. This refers also to some hydrogen-type atoms: hydrogen anti-atom, atom composed of proton and antiproton, and positronium. In a positronium (a hydrogen type atom, which consists of a positron and electron) the masses of both particles are equal. The charges of the parti ...
... which is present in the atom. This refers also to some hydrogen-type atoms: hydrogen anti-atom, atom composed of proton and antiproton, and positronium. In a positronium (a hydrogen type atom, which consists of a positron and electron) the masses of both particles are equal. The charges of the parti ...
The Vibrating String
... A glowing, low density gas, such as a neon sign, produces a discrete emission spectrum. This type of spectra is seen as having individual lines of different colors. The colors that are emitted by an excited atom are characteristic of that particular kind of atom. They represent a unique set of ener ...
... A glowing, low density gas, such as a neon sign, produces a discrete emission spectrum. This type of spectra is seen as having individual lines of different colors. The colors that are emitted by an excited atom are characteristic of that particular kind of atom. They represent a unique set of ener ...
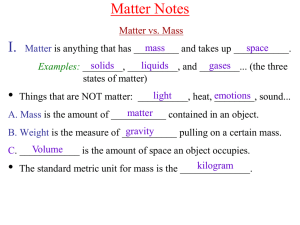
Chem A Week 2 Matter Notes
... chromatography. It is the physical separation of a mixture into its individual components. It involves using a solvent to pass through the mixture. What solvent should I use? The solvent used depends upon the solubility of the mixture you are trying to separate. Examples are water, isopropyl alcohol ...
... chromatography. It is the physical separation of a mixture into its individual components. It involves using a solvent to pass through the mixture. What solvent should I use? The solvent used depends upon the solubility of the mixture you are trying to separate. Examples are water, isopropyl alcohol ...
Worksheet - 1 - International Indian School, Riyadh
... 3. Calculate molecular mass of C6H12O6 molecule. 4. State the law of multiple proportion. 5. How many atoms of Calcium are there in 2g of Ca? 2 marks Question: 6. Define element, compound and mixture. 7. Define Gay Lussac’s law of gaseous volumes. Explain with one suitable example. 8. An organo meta ...
... 3. Calculate molecular mass of C6H12O6 molecule. 4. State the law of multiple proportion. 5. How many atoms of Calcium are there in 2g of Ca? 2 marks Question: 6. Define element, compound and mixture. 7. Define Gay Lussac’s law of gaseous volumes. Explain with one suitable example. 8. An organo meta ...
Particle Physics
... • Protons/nuclei can look point-like under many experimental conditions • Atoms/molecules can look point-like to a typical human QFT can be used to describe any such system… … it has nothing to do with the system being “fundamental” But QFT becomes essential when the energies are such that particles ...
... • Protons/nuclei can look point-like under many experimental conditions • Atoms/molecules can look point-like to a typical human QFT can be used to describe any such system… … it has nothing to do with the system being “fundamental” But QFT becomes essential when the energies are such that particles ...
Ch. 3 - Chemical Reactions
... two molecules of aqueous hydrochloric acid to produce one unit of aqueous zinc chloride and one molecule of hydrogen gas. ...
... two molecules of aqueous hydrochloric acid to produce one unit of aqueous zinc chloride and one molecule of hydrogen gas. ...
Light and quantized Energy Section 1
... Unique for each element and can be used to identify an element or determine whether an element is part of an unknown compound. ...
... Unique for each element and can be used to identify an element or determine whether an element is part of an unknown compound. ...
Chapter 9: Atoms
... These trend continue where elements that have similar chemical activity have a similar population in their outer electron shells. The chemical activity is to a great extent determined by the energy levels. ...
... These trend continue where elements that have similar chemical activity have a similar population in their outer electron shells. The chemical activity is to a great extent determined by the energy levels. ...
$doc.title
... plasma is initially in thermal equilibrium with the temperature T0 . The magnetic field is then slowly (compared to the particle gyro-‐frequencies) increased to 1 T , in such a way that particle collis ...
... plasma is initially in thermal equilibrium with the temperature T0 . The magnetic field is then slowly (compared to the particle gyro-‐frequencies) increased to 1 T , in such a way that particle collis ...
Chapter 2. The Chemical Context of Life
... Pair of electrons not shared equally by 2 atoms Water = O + H oxygen has stronger “attraction” for the shared electrons than hydrogen oxygen has higher ...
... Pair of electrons not shared equally by 2 atoms Water = O + H oxygen has stronger “attraction” for the shared electrons than hydrogen oxygen has higher ...
Photon model of light - High Point University
... spectrum and an emission spectrum; to apply conservation of energy to determine the energy of a photon emitted or a photon absorbed by a hydrogen atom. ...
... spectrum and an emission spectrum; to apply conservation of energy to determine the energy of a photon emitted or a photon absorbed by a hydrogen atom. ...
study guide first semester chemistry
... 1. Write the balanced equation for the following: (include the state of each reactant and product) a. magnesium reacts with nitrogen to produce magnesium nitride. (3Mg(s) + N2(g) Mg3N2(s) b. silver nitrate reacts with copper to form copper(II) nitrate and silver. ...
... 1. Write the balanced equation for the following: (include the state of each reactant and product) a. magnesium reacts with nitrogen to produce magnesium nitride. (3Mg(s) + N2(g) Mg3N2(s) b. silver nitrate reacts with copper to form copper(II) nitrate and silver. ...
QUIZ
... It’s not really very funny. It might be slightly humorous if one remembers that neutrons have no charge. 45. Can you tell me who Schroedinger's Cat is? (25 points) On June 7 of 1935, Erwin Schroedinger wrote to Albert Einstein to congratulate him on what is now known as the EPR paper, a famous probl ...
... It’s not really very funny. It might be slightly humorous if one remembers that neutrons have no charge. 45. Can you tell me who Schroedinger's Cat is? (25 points) On June 7 of 1935, Erwin Schroedinger wrote to Albert Einstein to congratulate him on what is now known as the EPR paper, a famous probl ...
Atomic and Molecular Physics for Physicists Ben-Gurion University of the Negev
... Interpreting the quantum world / Jeffrey Bub The undivided universe / David Bohm & Basil Hiley • Many of the 20th century physicists did not like QM. Some of them were indeed its fathers e.g. Einstein (photo electric effect) de-Broglie (the wave nature of paticles) and Schroedinger (the wave formali ...
... Interpreting the quantum world / Jeffrey Bub The undivided universe / David Bohm & Basil Hiley • Many of the 20th century physicists did not like QM. Some of them were indeed its fathers e.g. Einstein (photo electric effect) de-Broglie (the wave nature of paticles) and Schroedinger (the wave formali ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.