1.1. Atomic structure
... analysis is capable of reaching, we must admit as elements all the substances into which we are capable, by any means, to reduce bodies by decomposition. Not that we are entitled to affirm that these substances we consider as simple may not be compounded of two, or even of a greater number of princ ...
... analysis is capable of reaching, we must admit as elements all the substances into which we are capable, by any means, to reduce bodies by decomposition. Not that we are entitled to affirm that these substances we consider as simple may not be compounded of two, or even of a greater number of princ ...
IPhO 2016 - Theory - Large Hadron Collider
... In the following, the particles produced in a collision in a typical LHC detector are identified in a two stage detector, consisting of a tracking detector and a ToF detector. Figure 3 shows the setup of the two stage detector in the plane transverse and longitudinal to the proton beams. Both detecto ...
... In the following, the particles produced in a collision in a typical LHC detector are identified in a two stage detector, consisting of a tracking detector and a ToF detector. Figure 3 shows the setup of the two stage detector in the plane transverse and longitudinal to the proton beams. Both detecto ...
8.3 Metals - UNSW Chemistry
... mass of the middle member of each triad is very close to the average of the remaining members of the triad. Consider the atomic weights of the following four triads given below: ...
... mass of the middle member of each triad is very close to the average of the remaining members of the triad. Consider the atomic weights of the following four triads given below: ...
Short answers Short Problems
... frame. This is because we would measure the “birth” and decay (into something else) of the particle as events that happen at the same spot in that frame. In the Mamahuhu’s frame, the lifetime is 2.4×10−4 s . In a lab frame, the Mamahuhu’s frame is moving at a speed of 0.9c. The lifetime a scientist ...
... frame. This is because we would measure the “birth” and decay (into something else) of the particle as events that happen at the same spot in that frame. In the Mamahuhu’s frame, the lifetime is 2.4×10−4 s . In a lab frame, the Mamahuhu’s frame is moving at a speed of 0.9c. The lifetime a scientist ...
Document
... An elastic collision is a collision in which the total kinetic energy of the colliding bodies after collision is equal to their total kinetic energy before collision. Elastic collisions occur only if there is no conversion of kinetic energy into other forms, as in the collision of atoms. Inelastic c ...
... An elastic collision is a collision in which the total kinetic energy of the colliding bodies after collision is equal to their total kinetic energy before collision. Elastic collisions occur only if there is no conversion of kinetic energy into other forms, as in the collision of atoms. Inelastic c ...
Organic compounds are covalent compounds composed of carbon
... SPI 0807.9.4 – Differentiate between a mixture and a compound ...
... SPI 0807.9.4 – Differentiate between a mixture and a compound ...
Writing and Classifying Balanced Equations
... Write the word or words that best completes each sentence. balanced, product, separation, reactants, chemical reaction, or element ...
... Write the word or words that best completes each sentence. balanced, product, separation, reactants, chemical reaction, or element ...
Exam #: Printed Name: Signature: PHYSICS DEPARTMENT
... Find the eigenfunctions and eigenvalues of this system under this Hamiltonian. ...
... Find the eigenfunctions and eigenvalues of this system under this Hamiltonian. ...
Lect 23 Presentation
... The electrons in a large group of hydrogen atoms are excited to the n=3 level. How many spectral lines will be produced? ...
... The electrons in a large group of hydrogen atoms are excited to the n=3 level. How many spectral lines will be produced? ...
elements
... -NOBLE GAS(Inert Gases) The most stable e- arrangement. Normally do not react with other elements to form compounds ALL are gases and are found as single atoms in nature. ...
... -NOBLE GAS(Inert Gases) The most stable e- arrangement. Normally do not react with other elements to form compounds ALL are gases and are found as single atoms in nature. ...
Short answers Short Problems
... hit resulting in 9 more projectile neutrons ready to split other atoms, and so on. That’s the concept of a “chain-reaction”. Interestingly, the sum of the rest mass of the original particles (neutron plus U-235) is more than the sum of the rest mass of the outputted particles (Ba-141, Kr-92, and 3 n ...
... hit resulting in 9 more projectile neutrons ready to split other atoms, and so on. That’s the concept of a “chain-reaction”. Interestingly, the sum of the rest mass of the original particles (neutron plus U-235) is more than the sum of the rest mass of the outputted particles (Ba-141, Kr-92, and 3 n ...
The Franck-Hertz Experiment with Neon tube
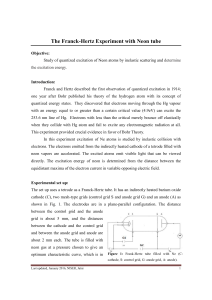
... one year after Bohr published his theory of the hydrogen atom with its concept of ...
... one year after Bohr published his theory of the hydrogen atom with its concept of ...
Thirteenth quantum mechanics sheet
... From b) it follows that it is possible to form joint Eigenvectors of J~2 , J3 , L these Eigenvectors |j, mj ; l, si with J~2 |j, mj ; l, si = h̄2 j(j + 1)|j, mj ; l, si J3 |j, mj ; l, si = h̄mj |j, mj ; l, si ~ 2 |j, mj ; l, si = h̄2 l(l + 1)|j, mj ; l, si L ~ 2 |j, mj ; l, si = h̄2 s(s + 1)|j, mj ; ...
... From b) it follows that it is possible to form joint Eigenvectors of J~2 , J3 , L these Eigenvectors |j, mj ; l, si with J~2 |j, mj ; l, si = h̄2 j(j + 1)|j, mj ; l, si J3 |j, mj ; l, si = h̄mj |j, mj ; l, si ~ 2 |j, mj ; l, si = h̄2 l(l + 1)|j, mj ; l, si L ~ 2 |j, mj ; l, si = h̄2 s(s + 1)|j, mj ; ...
Questions and Answers - hrsbstaff.ednet.ns.ca
... 1. Which of the coloured lights (red, orange, blue) on a Christmas tree emits photons with the most and least energy? Explain. 2. Does your stove emit energy when the burner is not turned on? Explain. 3. A single photon is ejected from a light source with a frequency of 2.0 x 1014 Hz. How much energ ...
... 1. Which of the coloured lights (red, orange, blue) on a Christmas tree emits photons with the most and least energy? Explain. 2. Does your stove emit energy when the burner is not turned on? Explain. 3. A single photon is ejected from a light source with a frequency of 2.0 x 1014 Hz. How much energ ...
Inverse mapping
... for the relationship between the energy gap and the frequency or wavelength If you want a different wavelength of light to be emitted, you need to find a different material. ...
... for the relationship between the energy gap and the frequency or wavelength If you want a different wavelength of light to be emitted, you need to find a different material. ...
Classification of Matter
... 1. Elements are the simplest form of matter. 2. Elements are the building blocks of all substances and cannot be easily divided into smaller subunits by ordinary chemical processes. 3. Elements are organized by atomic number on the periodic table. 4. Elements are identified by their symbols. ...
... 1. Elements are the simplest form of matter. 2. Elements are the building blocks of all substances and cannot be easily divided into smaller subunits by ordinary chemical processes. 3. Elements are organized by atomic number on the periodic table. 4. Elements are identified by their symbols. ...
Answers to Questions - EECS: www
... the effective mass of electrons and holes in semiconductors? A1: The first successful cyclotron resonance experiments on germanium (Ge) were published in 1953 by Dresselhaus, Kip and Kittel of the Physics Dept. at UC Berkeley. Soon afterwards (in 1954) Lax, Zeiger, and Rosenblum of MIT ...
... the effective mass of electrons and holes in semiconductors? A1: The first successful cyclotron resonance experiments on germanium (Ge) were published in 1953 by Dresselhaus, Kip and Kittel of the Physics Dept. at UC Berkeley. Soon afterwards (in 1954) Lax, Zeiger, and Rosenblum of MIT ...
Facilitator`s Guide PDF
... 4. Because two electrons can’t occupy the same quantum state, they fill from the “bottom up.” Electrons must fill the lowest n states, as these represent lower energy values. The lowerlvalues fill next, because those allow the electron to remain closer to the nucleus. So the 1s orbital fills first, ...
... 4. Because two electrons can’t occupy the same quantum state, they fill from the “bottom up.” Electrons must fill the lowest n states, as these represent lower energy values. The lowerlvalues fill next, because those allow the electron to remain closer to the nucleus. So the 1s orbital fills first, ...
Document
... number) with two possible values and formulated the Pauli exclusion principle. • 1925 – Ralph Kronig, George Uhlenbeck & Samuel Goudsmit – identified Pauli's new degree of freedom as electron spin and suggested a physical interpretation of particles spinning around their own axis. • 1926 – Enrico Fe ...
... number) with two possible values and formulated the Pauli exclusion principle. • 1925 – Ralph Kronig, George Uhlenbeck & Samuel Goudsmit – identified Pauli's new degree of freedom as electron spin and suggested a physical interpretation of particles spinning around their own axis. • 1926 – Enrico Fe ...
Atomic Units
... A note on Units You may have taken another theory course at some point, where “theory units” were used, that appeared to set all the fundamental constants equal to one. However, since the fine structure constant is always α = e2 /h̄c = 1/137, you can’t simultaneously set e, h̄, and c all equal to on ...
... A note on Units You may have taken another theory course at some point, where “theory units” were used, that appeared to set all the fundamental constants equal to one. However, since the fine structure constant is always α = e2 /h̄c = 1/137, you can’t simultaneously set e, h̄, and c all equal to on ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.