Chem. 121, Sec 11 Name: Student I.D. Please Show Your Work
... 8. Determine the relative rates of diffusion of hydrogen gas and oxygen gas at 25◦C? (3 marks) ...
... 8. Determine the relative rates of diffusion of hydrogen gas and oxygen gas at 25◦C? (3 marks) ...
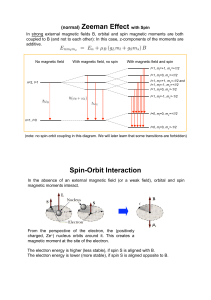
(normal) Zeeman Effect with Spin Spin
... 2s+1 : “Spin Multiplicity” " total number of spin states [e.g. 2s+1=2 (up and down) for s=1/2]. ...
... 2s+1 : “Spin Multiplicity” " total number of spin states [e.g. 2s+1=2 (up and down) for s=1/2]. ...
Lecture 24 (Slides) October 18
... • 1. Which of the following atoms and ions are paramagnetic (i.e. have unpaired electrons). Note: An even number of electrons does not indicate that all electrons are paired. (a) He atom, (b) F atom, (c) As atom, (d) F- ion (e) Al3+ ion and (f) Fe atom. • 2. Arrange the following in order of increas ...
... • 1. Which of the following atoms and ions are paramagnetic (i.e. have unpaired electrons). Note: An even number of electrons does not indicate that all electrons are paired. (a) He atom, (b) F atom, (c) As atom, (d) F- ion (e) Al3+ ion and (f) Fe atom. • 2. Arrange the following in order of increas ...
Electronic Structure of Atoms
... – Indicate whether energy is absorbed or emitted as the electron moves from n=4 to n=2. Explain (there are no calculations involved) – Determine the wavelength of the spectral line. – Indicate whether the wavelength calculated in the previous part is longer or shorter than the wavelength assoicated ...
... – Indicate whether energy is absorbed or emitted as the electron moves from n=4 to n=2. Explain (there are no calculations involved) – Determine the wavelength of the spectral line. – Indicate whether the wavelength calculated in the previous part is longer or shorter than the wavelength assoicated ...
Physics, Chapter 44: Stable Nuclei
... range of mass numbers runs from 1 to more than 250. The atomic masses of these isotopes differ very little from whole numbers. The number of stable isotopes per element varies from 1 for elements fluorine and gold to 10 for element tin. There is one thing which is common to all the isotopes of anyon ...
... range of mass numbers runs from 1 to more than 250. The atomic masses of these isotopes differ very little from whole numbers. The number of stable isotopes per element varies from 1 for elements fluorine and gold to 10 for element tin. There is one thing which is common to all the isotopes of anyon ...
4.quantumorbitals
... Quantum Theory The electron is like a cloud of negative energy or a wave. Orbitals are areas in 3D space where the electrons most probably are. The energy of the electron is in its vibrational modes- like notes on a guitar string. Photons are produced when high energy modes change to lower energy mo ...
... Quantum Theory The electron is like a cloud of negative energy or a wave. Orbitals are areas in 3D space where the electrons most probably are. The energy of the electron is in its vibrational modes- like notes on a guitar string. Photons are produced when high energy modes change to lower energy mo ...
1. What is the total number of electrons in the 2p
... ___ 78. Which atom has the largest radius? (1) Li; (2) Be; (3) C; (4) F. ___ 79. Isotopes are atoms which have different (1) atomic masses; (2) atomic numbers; (3) atomic radii; (4) electron configurations. ___ 80. When the aluminum atom is in the ground state then how many orbitals contain only one ...
... ___ 78. Which atom has the largest radius? (1) Li; (2) Be; (3) C; (4) F. ___ 79. Isotopes are atoms which have different (1) atomic masses; (2) atomic numbers; (3) atomic radii; (4) electron configurations. ___ 80. When the aluminum atom is in the ground state then how many orbitals contain only one ...
1. Select the correct statement about subatomic particles. a
... 23. Select the correct statement concerning formula C2H6O. a. It is a molecular formula. b. It is a formula unit. c. It gives information about molecular structure. d. It is the formula of an ionic compound. e. It represents a molecule made of 1 carbon atom, 2 hydrogen atoms, and 6 oxygen atoms. 24. ...
... 23. Select the correct statement concerning formula C2H6O. a. It is a molecular formula. b. It is a formula unit. c. It gives information about molecular structure. d. It is the formula of an ionic compound. e. It represents a molecule made of 1 carbon atom, 2 hydrogen atoms, and 6 oxygen atoms. 24. ...
1.2 Properties and Changes of Matter
... You must wear safety glasses for this lab. Please, be very careful with the chemicals. You may work in groups of three. ...
... You must wear safety glasses for this lab. Please, be very careful with the chemicals. You may work in groups of three. ...
Name Date Per ______ Physics – Std 5e: Electrostatics and
... (a) positive ion (b) negative ion (c) different element 4. To say that electric charge is conserved is to say that electric charge: (a) may occur in an infinite variety of quantities (b) is a whole number multiple of the charge of one electron (c) will interact with neighboring electric charges (d) ...
... (a) positive ion (b) negative ion (c) different element 4. To say that electric charge is conserved is to say that electric charge: (a) may occur in an infinite variety of quantities (b) is a whole number multiple of the charge of one electron (c) will interact with neighboring electric charges (d) ...
vuletic
... with time varying (RF) electric fields. These traps are limited in size and by micromotion, residual motion inherent in these RF traps. We are developing a new technique that uses an optical standing wave to stabilize and cool a linear array of ions. Our method also allows much finer spatial resolut ...
... with time varying (RF) electric fields. These traps are limited in size and by micromotion, residual motion inherent in these RF traps. We are developing a new technique that uses an optical standing wave to stabilize and cool a linear array of ions. Our method also allows much finer spatial resolut ...
PowerPoint
... Elements- simplest kind of matter Cannot be broken down All one kind of atom. Compounds are substances that can be broken down by chemical methods • When they are broken down, the pieces have completely different properties than the compound. • Made of molecules- two or more atoms ...
... Elements- simplest kind of matter Cannot be broken down All one kind of atom. Compounds are substances that can be broken down by chemical methods • When they are broken down, the pieces have completely different properties than the compound. • Made of molecules- two or more atoms ...
Zealey Phys-in-Cont
... Instead we average the individual wave packets and interpret them as a continuous wave. ...
... Instead we average the individual wave packets and interpret them as a continuous wave. ...
Electrons in Atoms
... electromagnetic radiation. This model is disastrous because it predicts that all atoms are unstable. To overcome this difficulty, Niels Bohr, in 1913, proposed that electrons could only have certain classical motions: ...
... electromagnetic radiation. This model is disastrous because it predicts that all atoms are unstable. To overcome this difficulty, Niels Bohr, in 1913, proposed that electrons could only have certain classical motions: ...
Solon City Schools
... • To determine the molar mass of an element, look on the table. • To determine the molar mass of a compound, add up the molar masses of the elements that make it up. ...
... • To determine the molar mass of an element, look on the table. • To determine the molar mass of a compound, add up the molar masses of the elements that make it up. ...
Chapter 2
... • To determine the molar mass of an element, look on the table. • To determine the molar mass of a compound, add up the molar masses of the elements that make it up. ...
... • To determine the molar mass of an element, look on the table. • To determine the molar mass of a compound, add up the molar masses of the elements that make it up. ...
Differentiate between a) Chemical vapor deposition (CVD) and
... The etch reactants are in a gas or vapor phase and mostly ionized The etching is due to both physical and chemical processes The ions being high energy particles are able to physically knock off the material without having to react with it ...
... The etch reactants are in a gas or vapor phase and mostly ionized The etching is due to both physical and chemical processes The ions being high energy particles are able to physically knock off the material without having to react with it ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.