Chemical and Molecular Formulas PPT
... “When carbon and oxygen form the compounds carbon monoxide and carbon dioxide, the different masses of carbon that combine with the same mass of oxygen are in the ratio of 2:1”? • law of multiple proportions ...
... “When carbon and oxygen form the compounds carbon monoxide and carbon dioxide, the different masses of carbon that combine with the same mass of oxygen are in the ratio of 2:1”? • law of multiple proportions ...
Motors and Generators
... Hertz observed that the spark between the gap in the transmitter loop caused an electrical disturbance between the gaps in the detecting loop. Hertz observed that the gap in the detector could be made larger and still generate sparks when the radiation from the transmitting spark shone directly into ...
... Hertz observed that the spark between the gap in the transmitter loop caused an electrical disturbance between the gaps in the detecting loop. Hertz observed that the gap in the detector could be made larger and still generate sparks when the radiation from the transmitting spark shone directly into ...
CHAPTER 2: THE ATOMS AND MOLECULES OF ANCIENT EARTH
... 2. Redox reactions are the most common chemical reactions in biology. 3. Reduction of carbon was a key step in chemical evolution. a. Carbon is the most versatile molecule found in biological tissues. (1) Each carbon atom can form four bonds with other molecules. (2) Carbon atoms form the skeleton o ...
... 2. Redox reactions are the most common chemical reactions in biology. 3. Reduction of carbon was a key step in chemical evolution. a. Carbon is the most versatile molecule found in biological tissues. (1) Each carbon atom can form four bonds with other molecules. (2) Carbon atoms form the skeleton o ...
CH30S Chemical Reactions Part 2 Unit Review
... 24. Calculate the volume of sulphur dioxide at STP that can be generated from 19.2g of iron (III) oxide reacting with excess sulphur according to the unbalanced reaction: S + Fe2O3 SO2 + Fe (4.0L SO2) 25. Calculate the volume of ammonia gas that could be produced from 9.5L of hydrogen gas and and ...
... 24. Calculate the volume of sulphur dioxide at STP that can be generated from 19.2g of iron (III) oxide reacting with excess sulphur according to the unbalanced reaction: S + Fe2O3 SO2 + Fe (4.0L SO2) 25. Calculate the volume of ammonia gas that could be produced from 9.5L of hydrogen gas and and ...
Chemistry I Exam
... E. The graph is a curve rather than a straight line because the speed of the bicycle decreased as the time of day increased. ...
... E. The graph is a curve rather than a straight line because the speed of the bicycle decreased as the time of day increased. ...
May 1998
... A Carnot engine uses n moles of an ideal gas as its working substance. The absolute temperatures of its hot and cold reservoirs are denoted by T1 and T2 , respectively. The net work performed by the engine in one cycle of operation is W. The specific heats of the gas may be assumed independent of th ...
... A Carnot engine uses n moles of an ideal gas as its working substance. The absolute temperatures of its hot and cold reservoirs are denoted by T1 and T2 , respectively. The net work performed by the engine in one cycle of operation is W. The specific heats of the gas may be assumed independent of th ...
Chemical Composition
... • If I want to know how many O2 molecules I will need or how many CO2 molecules I can make, I will need to know how many C atoms are in the sample of carbon I am starting with • Dalton used the percentages of elements in compounds and the chemical formulas to deduce the relative masses of atoms • Un ...
... • If I want to know how many O2 molecules I will need or how many CO2 molecules I can make, I will need to know how many C atoms are in the sample of carbon I am starting with • Dalton used the percentages of elements in compounds and the chemical formulas to deduce the relative masses of atoms • Un ...
Serge Haroche
... The Nobel Laureates have opened the door to a new era of experimentation with quantum physics by demonstrating the direct observation of individual quantum particles without destroying them. For single particles of light or matter the laws of classical physics cease to apply and quantum physics take ...
... The Nobel Laureates have opened the door to a new era of experimentation with quantum physics by demonstrating the direct observation of individual quantum particles without destroying them. For single particles of light or matter the laws of classical physics cease to apply and quantum physics take ...
- Catalyst
... Question 7: Fill in the blanks of the statements below with the words in the box. Note, you will only use each word once. A. atom ...
... Question 7: Fill in the blanks of the statements below with the words in the box. Note, you will only use each word once. A. atom ...
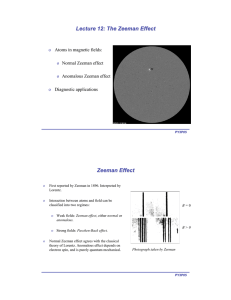
o Atoms in magnetic fields: Normal Zeeman effect Anomalous Zeeman effect
... Filled shells have no net spin, so only consider valence electrons. Since electrons have spin 1/2, not possible to obtain S = 0 from atoms with odd number of valence electrons. ...
... Filled shells have no net spin, so only consider valence electrons. Since electrons have spin 1/2, not possible to obtain S = 0 from atoms with odd number of valence electrons. ...
104 Homework Packet - Rogue Community College
... According to Le Chatelier’s Principle, adding reactants (or removing products) drives the equilibrium to the __________, adding products (or removing reactants) drives the equilibrium to the __________, increasing temperature favors the ___________________ reaction, decreasing temperature favors the ...
... According to Le Chatelier’s Principle, adding reactants (or removing products) drives the equilibrium to the __________, adding products (or removing reactants) drives the equilibrium to the __________, increasing temperature favors the ___________________ reaction, decreasing temperature favors the ...
MODERN PHYSICS CET questions from Bohr`s atom model
... 2. Strokes lines are more intense than the antistokes lines 3. The intensity of stokes lines is found to depend on temperature 4. Stokes and antistokes lines are polarized 52. Pick out the incorrect statement from the following : 1. Rayleigh scattering is coherent scattering 2. Raman effect is incoh ...
... 2. Strokes lines are more intense than the antistokes lines 3. The intensity of stokes lines is found to depend on temperature 4. Stokes and antistokes lines are polarized 52. Pick out the incorrect statement from the following : 1. Rayleigh scattering is coherent scattering 2. Raman effect is incoh ...
Ch.41- Orbital angular momentum, counting states
... momentum has the same magnitude L for each these five states, but has different values of the zcomponent Lz. Copyright © 2012 Pearson Education Inc. ...
... momentum has the same magnitude L for each these five states, but has different values of the zcomponent Lz. Copyright © 2012 Pearson Education Inc. ...
Nuclear Physics
... In fact the nucleus could decay first to the 0.013 level, and then the ground state, thus emitting two gamma photons. ...
... In fact the nucleus could decay first to the 0.013 level, and then the ground state, thus emitting two gamma photons. ...
Distribution of Atomic Ionization Potentials
... atoms, dotted lines in case of interpolation when data is missing between two measures. The different atomic structures are enhanced for a better visibility. ...
... atoms, dotted lines in case of interpolation when data is missing between two measures. The different atomic structures are enhanced for a better visibility. ...
P. LeClair
... kinetic energy, and both particles lose the same potential energy by moving through the potential difference. Both particles have equal but opposite charges and move through equal and opposite potential differences – since the negatively charged electron moves through a positive potential difference ...
... kinetic energy, and both particles lose the same potential energy by moving through the potential difference. Both particles have equal but opposite charges and move through equal and opposite potential differences – since the negatively charged electron moves through a positive potential difference ...
AP Atomics Class Packet Unit 2 - Ms. Drury`s Flipped Chemistry
... o 1.A.2 Chemical analysis provides a method for determining the relative number of atoms in a substance, which can be used to identify the substance or determine purity. o 1.D.2 An early model of the atom stated that all atoms of an element are identical. Mass spectrometry data demonstrate evidence ...
... o 1.A.2 Chemical analysis provides a method for determining the relative number of atoms in a substance, which can be used to identify the substance or determine purity. o 1.D.2 An early model of the atom stated that all atoms of an element are identical. Mass spectrometry data demonstrate evidence ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.