Solutions Intro
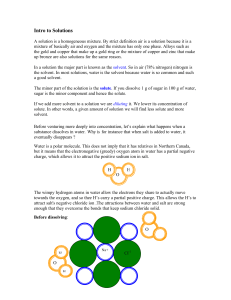
... If we add more solvent to a solution we are diluting it. We lower its concentration of solute. In other words, a given amount of solution we will find less solute and more solvent. Before venturing more deeply into concentration, let’s explain what happens when a substance dissolves in water. Why is ...
... If we add more solvent to a solution we are diluting it. We lower its concentration of solute. In other words, a given amount of solution we will find less solute and more solvent. Before venturing more deeply into concentration, let’s explain what happens when a substance dissolves in water. Why is ...
Atomic/Nuclear Models
... atom must be an integral multiple of the electronic charge and, since atoms are electrically neutral, the positive and negative charges must be numerically equal. The emission of electrons from atoms under widely varying conditions was convincing evidence that electrons exist as such inside atoms. ...
... atom must be an integral multiple of the electronic charge and, since atoms are electrically neutral, the positive and negative charges must be numerically equal. The emission of electrons from atoms under widely varying conditions was convincing evidence that electrons exist as such inside atoms. ...
Chapter 6 Electronic Structure of Atoms
... 2. Electrons in permitted orbits have specific, “allowed” energies; these energies will not be radiated from the atom. Electronic Structure of Atoms ...
... 2. Electrons in permitted orbits have specific, “allowed” energies; these energies will not be radiated from the atom. Electronic Structure of Atoms ...
Elements and Compounds
... the atmosphere AND differentiate between elements and compounds on the most basic level. ...
... the atmosphere AND differentiate between elements and compounds on the most basic level. ...
Preview to Mole Activity #2 preview_to_mole_activity_21
... Today’s activity will introduce you to a unit of measure without which chemistry would not exist. It is a unit much like a dozen, which helps us count things. “Why would a chemist need to count things?” you might ask. Examine the following chemical equation: 2H2 + O2 ------> 2H2O This can be interpr ...
... Today’s activity will introduce you to a unit of measure without which chemistry would not exist. It is a unit much like a dozen, which helps us count things. “Why would a chemist need to count things?” you might ask. Examine the following chemical equation: 2H2 + O2 ------> 2H2O This can be interpr ...
Physical Science CP Seton Hall Preparatory School Mr. Greene
... Short-wave solar radiation vs. long-wave terrestrial radiation CO2, water vapor, and methane as greenhouse gases The Greenhouse Effect and Global Warming Planetary albedo Pressure-gradient force Coriolis force Cloud types Convectional lifting Cold front/Warm front (types of precipitation and conditi ...
... Short-wave solar radiation vs. long-wave terrestrial radiation CO2, water vapor, and methane as greenhouse gases The Greenhouse Effect and Global Warming Planetary albedo Pressure-gradient force Coriolis force Cloud types Convectional lifting Cold front/Warm front (types of precipitation and conditi ...
Chapter 7 -- Radiative Corrections: some formal developments Chapter 7:
... W hen it seemed that about hydrogen atom we knew almost everything in 1947 W.E. Lamb and R.C. Retherford decided to check results of Dirac. They used microwaves technique, available from the constructions of radar The Lamb's shift*, a minimal difference in lowest energetic level of the excited hydro ...
... W hen it seemed that about hydrogen atom we knew almost everything in 1947 W.E. Lamb and R.C. Retherford decided to check results of Dirac. They used microwaves technique, available from the constructions of radar The Lamb's shift*, a minimal difference in lowest energetic level of the excited hydro ...
Review - Final Exam
... pure substances? Explain. How can the other term apply to substances and mixtures? Use examples to explain why. 7. What is the difference between: an element and a compound, an element and an atom, a compound and a molecule, & an element and an ion? Is it possible to have a molecule of an element? E ...
... pure substances? Explain. How can the other term apply to substances and mixtures? Use examples to explain why. 7. What is the difference between: an element and a compound, an element and an atom, a compound and a molecule, & an element and an ion? Is it possible to have a molecule of an element? E ...
The Electrical Conductivity of a Partially Ionized Argon
... plasma. The distribution function is expanded according to LAGUERRE polynomials up to the order of 3 . In this order the electrical conductivity of a LORENTZ gas, which is known exactly, is obtained to an accuracy of roughly 5%. The approximation tested in this way was then used to calculate the con ...
... plasma. The distribution function is expanded according to LAGUERRE polynomials up to the order of 3 . In this order the electrical conductivity of a LORENTZ gas, which is known exactly, is obtained to an accuracy of roughly 5%. The approximation tested in this way was then used to calculate the con ...
chemI.final.rev.probs
... 41. For the following reaction: 2K(s) + 2H2O(l) → 2KOH(aq) + H2(g) a) Determine the number of grams of KOH that will be produced when 97 g of potassium are used. b) Determine the number of liters of H2 gas that will be produced when 6.5 X 1024 molecules of water are reacted. ...
... 41. For the following reaction: 2K(s) + 2H2O(l) → 2KOH(aq) + H2(g) a) Determine the number of grams of KOH that will be produced when 97 g of potassium are used. b) Determine the number of liters of H2 gas that will be produced when 6.5 X 1024 molecules of water are reacted. ...
Unit 1 CHEMISTRY OVERVIEW: Matter and Measurement Important
... 15. Matter can be classified as a pure substance or a mixture. Pure substances can be elements or compounds. Compounds have to include more than one type of element (H2O). Compounds differ from mixtures in that they are chemically bonded through a chemical reaction. Elements may exist as single atom ...
... 15. Matter can be classified as a pure substance or a mixture. Pure substances can be elements or compounds. Compounds have to include more than one type of element (H2O). Compounds differ from mixtures in that they are chemically bonded through a chemical reaction. Elements may exist as single atom ...
STRUCTURE OF A TURE OF A TURE OF ATOM STRUCTURE OF A
... possess to eject an electron from a metal. It is different for different metals. When a photon of frequency 1.0×1015 s–1 was allowed to hit a metal surface, an electron having 1.988 × 10–19 J of kinetic energy was emitted. Calculate the threshold frequency of this metal. Show that an electron will n ...
... possess to eject an electron from a metal. It is different for different metals. When a photon of frequency 1.0×1015 s–1 was allowed to hit a metal surface, an electron having 1.988 × 10–19 J of kinetic energy was emitted. Calculate the threshold frequency of this metal. Show that an electron will n ...
Document
... Atoms are stable. Essentially all atoms have remained unchanged for billions of years. Atoms combine with each other. Atoms stick together to form molecules and stack up to form rigid solids. Even though atoms are mostly empty space, their interactions allow you to stand on a floor without falling t ...
... Atoms are stable. Essentially all atoms have remained unchanged for billions of years. Atoms combine with each other. Atoms stick together to form molecules and stack up to form rigid solids. Even though atoms are mostly empty space, their interactions allow you to stand on a floor without falling t ...
Quantum Chemistry Chem 6010 Fall 2016 Instructor: Steve Scheiner
... Students will learn to do the following: Use quantum reasoning to explain microscopic model processes, including particle in a box, harmonic oscillator and atoms. Formulate and use quantum mechanical operators. Explain and use the variation theorem and perturbation theory. Apply principles of electr ...
... Students will learn to do the following: Use quantum reasoning to explain microscopic model processes, including particle in a box, harmonic oscillator and atoms. Formulate and use quantum mechanical operators. Explain and use the variation theorem and perturbation theory. Apply principles of electr ...
Final Exam Review Guide
... 4. When it comes to evaluating a solute/solvent relationship, remember the phrase “like dissolves like.” Atoms/Periodic Table Units 1. Ionic compounds form when atoms gain or lose electrons. Metals lose electrons, nonmetals gain them. 2. The number of electrons gained or lost can be predicted with a ...
... 4. When it comes to evaluating a solute/solvent relationship, remember the phrase “like dissolves like.” Atoms/Periodic Table Units 1. Ionic compounds form when atoms gain or lose electrons. Metals lose electrons, nonmetals gain them. 2. The number of electrons gained or lost can be predicted with a ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.