HYDROGEN PEROXIDE 20%-50%
... The information and recommendations provided in this Material Safety Data Sheet are to the best of Miles Chemical Co.’s knowledge and belief accurate and reliable as of the date issued and was obtained from sources believed to be reliable. Miles Chemical Co. does not warrant or guarantee their accur ...
... The information and recommendations provided in this Material Safety Data Sheet are to the best of Miles Chemical Co.’s knowledge and belief accurate and reliable as of the date issued and was obtained from sources believed to be reliable. Miles Chemical Co. does not warrant or guarantee their accur ...
Review Package
... 26) a) If apple juice has a pH of 5 and vinegar has a pH of 3, which is more acidic? __________________ b) How many more hydrogen ions are there in the more acidic substance? ____________________ 27) How much more acidic is a solution with a pH of 4.5 than a solution with a pH of a) 5.5? b) 6.5? 28) ...
... 26) a) If apple juice has a pH of 5 and vinegar has a pH of 3, which is more acidic? __________________ b) How many more hydrogen ions are there in the more acidic substance? ____________________ 27) How much more acidic is a solution with a pH of 4.5 than a solution with a pH of a) 5.5? b) 6.5? 28) ...
Review Packet - Newton.k12.ma.us
... 5. - The molecular weight is the sum of the atomic weights of the atoms in a molecule of a compound. - The formula weight is the sum of the atomic weights of the atoms in a formula unit. - The molecular mass is the mass of one mole of any substance. 6. The advantage of using moles is that the quanti ...
... 5. - The molecular weight is the sum of the atomic weights of the atoms in a molecule of a compound. - The formula weight is the sum of the atomic weights of the atoms in a formula unit. - The molecular mass is the mass of one mole of any substance. 6. The advantage of using moles is that the quanti ...
Chem 150 Answer Key Problem Introductory Quantum Chemistry 1
... a) In Bohr’s model the electron orbits the nucleus. Both its position (e.g. radius from the nucleus) and its energy are precisely known (violation of Heisenberg’s uncertainty principle). In quantum mechanics the electron is in an orbital and not in an orbit. The most likely distance to find the elec ...
... a) In Bohr’s model the electron orbits the nucleus. Both its position (e.g. radius from the nucleus) and its energy are precisely known (violation of Heisenberg’s uncertainty principle). In quantum mechanics the electron is in an orbital and not in an orbit. The most likely distance to find the elec ...
ppt - plutonium
... How do we describe and apply the concept of electric field? How do we calculate the magnitude and direction of the force on a positive or negative charge in an electric field? How do we analyze the motion of a particle of known mass and charge in a uniform electric field? ...
... How do we describe and apply the concept of electric field? How do we calculate the magnitude and direction of the force on a positive or negative charge in an electric field? How do we analyze the motion of a particle of known mass and charge in a uniform electric field? ...
Chemical Equations
... • MS-PS1-5. I can explain the conservation of mass through a model of chemical reactions. • MS-PS1-3 I can gather information to describe the origins and impacts of synthetic material ...
... • MS-PS1-5. I can explain the conservation of mass through a model of chemical reactions. • MS-PS1-3 I can gather information to describe the origins and impacts of synthetic material ...
Chemical Changes and Structure Homework Booklet
... 12Mg are two different kinds of magnesium atom. a. What word is used to describe these types of atoms? b. Explain why they can be regarded as atoms of the same element? c. The relative atomic mass of magnesium is 24.3. What does this tell you about the relative amounts of each atom? An atom has atom ...
... 12Mg are two different kinds of magnesium atom. a. What word is used to describe these types of atoms? b. Explain why they can be regarded as atoms of the same element? c. The relative atomic mass of magnesium is 24.3. What does this tell you about the relative amounts of each atom? An atom has atom ...
Optically polarized atoms_ch_2
... In this approximation, energy of a configuration is just sum of Ei No reference to projections of li or to spins degeneracy If we go beyond the central-field approximation some of the degeneracies will be lifted Also spin-orbit (ls) interaction lifts some degeneracies In general, both effects nee ...
... In this approximation, energy of a configuration is just sum of Ei No reference to projections of li or to spins degeneracy If we go beyond the central-field approximation some of the degeneracies will be lifted Also spin-orbit (ls) interaction lifts some degeneracies In general, both effects nee ...
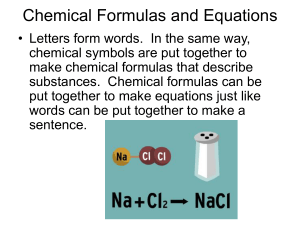
Chemical Formulas and Equations
... Chemical Formulas and Equations • Letters form words. In the same way, chemical symbols are put together to make chemical formulas that describe substances. Chemical formulas can be put together to make equations just like words can be put together to make a ...
... Chemical Formulas and Equations • Letters form words. In the same way, chemical symbols are put together to make chemical formulas that describe substances. Chemical formulas can be put together to make equations just like words can be put together to make a ...
isotopes, relative atomic mass and mass
... atoms are too small to be weighed directly, (although their masses can be determined indirectly by measuring their effect on each other). Atoms are so tiny that knowing the mass of any individual atom isn’t very useful, as chemists never carry out any experimental or practical work on just one atom ...
... atoms are too small to be weighed directly, (although their masses can be determined indirectly by measuring their effect on each other). Atoms are so tiny that knowing the mass of any individual atom isn’t very useful, as chemists never carry out any experimental or practical work on just one atom ...
Algebra - Militant Grammarian
... 3. A car engine working at a rate of 24 618 W is moving the car at a rate of 45 mph. What force is the engine exerting? 4. A star of mass 2.0 x 1030 kg moving with a velocity of 2.0 x 104 m/sec collides with a second star of mass 5.0 x 1030 kg moving with a velocity of 3.0 x 104 m/s in a direction a ...
... 3. A car engine working at a rate of 24 618 W is moving the car at a rate of 45 mph. What force is the engine exerting? 4. A star of mass 2.0 x 1030 kg moving with a velocity of 2.0 x 104 m/sec collides with a second star of mass 5.0 x 1030 kg moving with a velocity of 3.0 x 104 m/s in a direction a ...
*6th Grade Science-Chapter 5 Study Guide Lesson 5.1: Observing
... *6th Grade Science-Chapter 5 Study Guide Lesson 5.1: Observing Chemical Change *Changes in matter can be described in terms of physical changes and chemical changes. *Chemical reactions involve changes in properties and changes in energy that you can often observe. Physical change-any change that al ...
... *6th Grade Science-Chapter 5 Study Guide Lesson 5.1: Observing Chemical Change *Changes in matter can be described in terms of physical changes and chemical changes. *Chemical reactions involve changes in properties and changes in energy that you can often observe. Physical change-any change that al ...
2010 - The Physics Teacher
... (iii) What are X-rays? X-rays are electromagnetic radiation of high frequency / short wavelength (iv) How do they differ from light rays? X-rays penetrate matter / cause ionization. (v) Give two uses of X-rays. (Medical) analysis of bone structure/ luggage scanners (at airports) / any specific medic ...
... (iii) What are X-rays? X-rays are electromagnetic radiation of high frequency / short wavelength (iv) How do they differ from light rays? X-rays penetrate matter / cause ionization. (v) Give two uses of X-rays. (Medical) analysis of bone structure/ luggage scanners (at airports) / any specific medic ...
Chapter 3. Stoichiometry: Calculations with Chemical Formulas and
... (6.022 x 1023) of “building blocks” (atoms for most elements, molecules for molecular substances, formula units for ionic substances) in the same fashion as a dozen means 12 (eggs, people, items, etc.) Be able to appreciate how important the concept of a mole is Limiting reagents concept can be diff ...
... (6.022 x 1023) of “building blocks” (atoms for most elements, molecules for molecular substances, formula units for ionic substances) in the same fashion as a dozen means 12 (eggs, people, items, etc.) Be able to appreciate how important the concept of a mole is Limiting reagents concept can be diff ...
3 Fundamentals of Planetary Materials
... As we discussed in the last chapter, the abundances of elements are determined by nuclear physics. However, most elements are not stable in elemental form but are found as molecules or in compounds. Additionally, it is important to understand the difference between the chemistry of matter and its ph ...
... As we discussed in the last chapter, the abundances of elements are determined by nuclear physics. However, most elements are not stable in elemental form but are found as molecules or in compounds. Additionally, it is important to understand the difference between the chemistry of matter and its ph ...
Forces of the Universe - The Federation of Galaxy Explorers
... in a force field describe how an object will move when placed near an object that emits a force. The Unified Theory For many years, scientists have been trying to find a way to tie the four forces together into one theory. This is called the ‘Unified’ theory. To date, there has been no way to tie th ...
... in a force field describe how an object will move when placed near an object that emits a force. The Unified Theory For many years, scientists have been trying to find a way to tie the four forces together into one theory. This is called the ‘Unified’ theory. To date, there has been no way to tie th ...
Coupling Charged Particles to the Electromagnetic Field
... In this light, one can understand the Dirac quantization condition for electric charge. We have seen that if monopoles exist, they are described by singular field configurations. This singularity is seemingly a gauge artifact. It can be chosen, for example, to lie in different directions by making ...
... In this light, one can understand the Dirac quantization condition for electric charge. We have seen that if monopoles exist, they are described by singular field configurations. This singularity is seemingly a gauge artifact. It can be chosen, for example, to lie in different directions by making ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.