phase diagrams and IMF
... 4.) Use the phase diagram for water (above). It is not drawn to scale. The triple point occurs at 0.0060 atm and 0.01oC. Using this knowledge, answer the following questions: a. Label the triple point – use this as a reference point for the following questions (put a dot ...
... 4.) Use the phase diagram for water (above). It is not drawn to scale. The triple point occurs at 0.0060 atm and 0.01oC. Using this knowledge, answer the following questions: a. Label the triple point – use this as a reference point for the following questions (put a dot ...
Atoms and Term Symbols
... • physical cause: shielding is worst in this case.. a tricky quantum mechanics problem • Second rule: For a given spin configuration, the state with the largest L will have the lowest energy • physical cause: electrons orbiting in ‘opposite directions’ tend to bump into each other more often, thus m ...
... • physical cause: shielding is worst in this case.. a tricky quantum mechanics problem • Second rule: For a given spin configuration, the state with the largest L will have the lowest energy • physical cause: electrons orbiting in ‘opposite directions’ tend to bump into each other more often, thus m ...
Chemistry Spring Final Review
... a substance. B. A device used to measure the amount of heat absorbed or released during chemical or physical processes. C. Energy that always flows from a warmer object to a cooler object (high concentration to lower concentration). D. In any chemical or physical process, energy is neither created n ...
... a substance. B. A device used to measure the amount of heat absorbed or released during chemical or physical processes. C. Energy that always flows from a warmer object to a cooler object (high concentration to lower concentration). D. In any chemical or physical process, energy is neither created n ...
Physical Science
... • A static discharge is a transfer of charge between two objects because of a buildup of static electricity. • A thundercloud is a mighty generator of static electricity. As air masses move and swirl in the cloud, areas of positive and negative charge build up. ...
... • A static discharge is a transfer of charge between two objects because of a buildup of static electricity. • A thundercloud is a mighty generator of static electricity. As air masses move and swirl in the cloud, areas of positive and negative charge build up. ...
AP Chemistry
... all of the polyatomic ions memorized by the first day of school. All of this material should be review. AP Chemistry is a second year course for a reason. If there are problems in the packet that you are having difficulty with, do not panic. Contact me through email or some fellow classmates, so tha ...
... all of the polyatomic ions memorized by the first day of school. All of this material should be review. AP Chemistry is a second year course for a reason. If there are problems in the packet that you are having difficulty with, do not panic. Contact me through email or some fellow classmates, so tha ...
Chem 111 Summer 2003 Exam I Whelan Some Useful And Not So
... Metal, Non Metal, Halide, Noble Gas, Alkali Metal, Alkali Earth Metal, Transition Metal, Lanthanide or Actinide. ...
... Metal, Non Metal, Halide, Noble Gas, Alkali Metal, Alkali Earth Metal, Transition Metal, Lanthanide or Actinide. ...
Pg 65 The student`s spreadsheet is shown in Fig. 12.5. A B C D 1
... 11. Which graph shows the relationship between the pressure and volume of a fixed mass of gas at constant temperature? -insert diagram12. Assuming the temperature remains constant, which combination correctly describes the volume and the shape of a gas or liquid? ...
... 11. Which graph shows the relationship between the pressure and volume of a fixed mass of gas at constant temperature? -insert diagram12. Assuming the temperature remains constant, which combination correctly describes the volume and the shape of a gas or liquid? ...
Are quantum particles objects? - General Guide To Personal and
... where '; ; are 1 particle vectors. Pretty evidently, it does not specify which particle is in which state - there is no such determinate rule here. It is like the symmetrized triadic ‘the …rst particle is in the state ‘const. '’ the second in the state ‘const. ’, the third in the state ‘const. ’, or ...
... where '; ; are 1 particle vectors. Pretty evidently, it does not specify which particle is in which state - there is no such determinate rule here. It is like the symmetrized triadic ‘the …rst particle is in the state ‘const. '’ the second in the state ‘const. ’, the third in the state ‘const. ’, or ...
10. Quantitative Chemistry
... Atom economy is another measure of the efficiency of a chemical reaction. It is the amount of starting materials that end up as useful products. In an ideal chemical process, all the starting materials end up as useful products and no atom is wasted. If most of the starting materials end up as usefu ...
... Atom economy is another measure of the efficiency of a chemical reaction. It is the amount of starting materials that end up as useful products. In an ideal chemical process, all the starting materials end up as useful products and no atom is wasted. If most of the starting materials end up as usefu ...
Classification of
... c) _________compound_________________ - 2 or more elements whose atoms have chemically combined d) ____________mixture_____________ - 2 or more substances physically combined e) ______heterogeneous_________________ - mixture with individual parts visible f) _______states of matter___________________ ...
... c) _________compound_________________ - 2 or more elements whose atoms have chemically combined d) ____________mixture_____________ - 2 or more substances physically combined e) ______heterogeneous_________________ - mixture with individual parts visible f) _______states of matter___________________ ...
Luminescence model with quantum impact parameter for low energy ions H.S. Cruz-Galindo
... We have modified an analytical model of induced light production by energetic ions interacting in scintillating materials. The original model is based on the distribution of energy deposited by secondary electrons produced along the ion’s track. The range of scattered electrons, and thus the energy d ...
... We have modified an analytical model of induced light production by energetic ions interacting in scintillating materials. The original model is based on the distribution of energy deposited by secondary electrons produced along the ion’s track. The range of scattered electrons, and thus the energy d ...
Physics 200 Class #1 Outline
... Problem: The peak of the blackbody curve is measured to be at 1000 nm for a temperature of 2900 K. Find the temperature of the surface of the sun if the peak of the solar spectrum is at 500 nm. And now, the beginning of quantum mechanics: All attempts to predict the blackbody curve using classical p ...
... Problem: The peak of the blackbody curve is measured to be at 1000 nm for a temperature of 2900 K. Find the temperature of the surface of the sun if the peak of the solar spectrum is at 500 nm. And now, the beginning of quantum mechanics: All attempts to predict the blackbody curve using classical p ...
solutions for chapter 21 problems 4, 12, 19, 25, 33, 40, 50, 75, 89, 96.
... IDENTIFY: The net force on each charge must be zero. SET UP: The force diagram for the 6.50 C charge is given in Figure 21.40. FE is the force exerted on the charge by the uniform electric field. The charge is negative and the field is to the right, so the force exerted by the field is to the lef ...
... IDENTIFY: The net force on each charge must be zero. SET UP: The force diagram for the 6.50 C charge is given in Figure 21.40. FE is the force exerted on the charge by the uniform electric field. The charge is negative and the field is to the right, so the force exerted by the field is to the lef ...
Chemistry Unit Outcomes
... Outline who James Chadwick was and explain what Chadwick discovered. Precisely, explain the characteristics of protons, neutrons and electrons and where they are located in terms of the atom. Explain why protons are especially significant or important. List an example. Outline why an atom has no net ...
... Outline who James Chadwick was and explain what Chadwick discovered. Precisely, explain the characteristics of protons, neutrons and electrons and where they are located in terms of the atom. Explain why protons are especially significant or important. List an example. Outline why an atom has no net ...
AP Physics – Them Laws – 5
... (c) Determine the value of the suspended mass M that allows blocks 1 and 2 to move with constant velocity down the plane. ...
... (c) Determine the value of the suspended mass M that allows blocks 1 and 2 to move with constant velocity down the plane. ...
Astronomy
... Explain the relationship between momentum and force. State Newton’s second law of motion in terms of momentum. Calculate momentum given mass and velocity. Bill Nye – Momentum 8.2. Impulse Define impulse. Describe effects of impulses in everyday life. Determine the average effective force ...
... Explain the relationship between momentum and force. State Newton’s second law of motion in terms of momentum. Calculate momentum given mass and velocity. Bill Nye – Momentum 8.2. Impulse Define impulse. Describe effects of impulses in everyday life. Determine the average effective force ...
Balancing Chemical Equations Lab
... 1. Using your set of cards, replicate the chemical equation onto your desk. Record the following results into Table 1: 2. Identify the elements on the reactant side. 3. Count the number of atoms for each element. 4. Identify the elements on the product side. 5. Count the number of atoms on the produ ...
... 1. Using your set of cards, replicate the chemical equation onto your desk. Record the following results into Table 1: 2. Identify the elements on the reactant side. 3. Count the number of atoms for each element. 4. Identify the elements on the product side. 5. Count the number of atoms on the produ ...
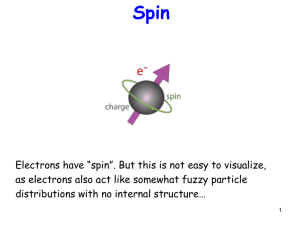
Slide1
... Spin is like angular momentum Recall m can have (2l+1) values between –l and l. For spin, since only 2 ...
... Spin is like angular momentum Recall m can have (2l+1) values between –l and l. For spin, since only 2 ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.