Section 7.5 Quantum Mechanics and the Atom
... physics to explain the behavior of the atom. • This process had already begun with the breaking down of the barrier between light as a wave and matter as a particle. • Einstein showed that light behaves as a particle • The Bohr model is the beginning to the process of treating matter (electrons) as ...
... physics to explain the behavior of the atom. • This process had already begun with the breaking down of the barrier between light as a wave and matter as a particle. • Einstein showed that light behaves as a particle • The Bohr model is the beginning to the process of treating matter (electrons) as ...
Chapter 07 and 08 Chemical Bonding and Molecular
... • Pure substance • Made of 2 or more elements in a definite proportion by mass • Physically and chemically different from the elements that make up the compound • All elements (except Noble gases) react to gain a stable octet. (duet-for H through B) • Compounds form to gain a stable valence shell wh ...
... • Pure substance • Made of 2 or more elements in a definite proportion by mass • Physically and chemically different from the elements that make up the compound • All elements (except Noble gases) react to gain a stable octet. (duet-for H through B) • Compounds form to gain a stable valence shell wh ...
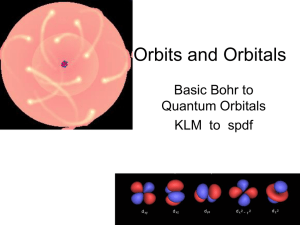
Orbits and Orbitals
... • Orbitals are half filled (with spins in the same direction) before they are doubly filled. • Orbitals are filled from lowest energy to highest energy. ...
... • Orbitals are half filled (with spins in the same direction) before they are doubly filled. • Orbitals are filled from lowest energy to highest energy. ...
linear momentum
... • Now we again examine the behaviour of the billiard balls. • Once the cue ball has begun to roll, no net external force acts on the (two-ball) system. • Thus Fnet = 0, and acom = 0 . • Acceleration = the rate of change in velocity, • thus the velocity of the centre of mass does not change. • When ...
... • Now we again examine the behaviour of the billiard balls. • Once the cue ball has begun to roll, no net external force acts on the (two-ball) system. • Thus Fnet = 0, and acom = 0 . • Acceleration = the rate of change in velocity, • thus the velocity of the centre of mass does not change. • When ...
Student Text, pp. 378-381
... Build your own versorium. Charge several objects and try your device. Also try it on an operating television screen. Examine the effect of turning the television off and on while keeping your versorium near the screen. Write a short report on your findings. 37. Design an experiment that can be used ...
... Build your own versorium. Charge several objects and try your device. Also try it on an operating television screen. Examine the effect of turning the television off and on while keeping your versorium near the screen. Write a short report on your findings. 37. Design an experiment that can be used ...
File 06_lecture
... • Erwin Schrödinger developed a mathematical treatment into which both the wave and particle nature of matter could be incorporated. • This is known as quantum mechanics. © 2012 Pearson Education, Inc. ...
... • Erwin Schrödinger developed a mathematical treatment into which both the wave and particle nature of matter could be incorporated. • This is known as quantum mechanics. © 2012 Pearson Education, Inc. ...
dicke-july2013x
... • We’ve incorporated CoM coordinates into , the “cooperation” operator; does not commute with ! • Thus, these are not stationary eigenstates of . • Classically, relative motion of radiators causes decoherence, but radiators with a common velocity will not decohere. • Quantum mechanically, analogous ...
... • We’ve incorporated CoM coordinates into , the “cooperation” operator; does not commute with ! • Thus, these are not stationary eigenstates of . • Classically, relative motion of radiators causes decoherence, but radiators with a common velocity will not decohere. • Quantum mechanically, analogous ...
The photoelectric effect - University of Toronto Physics
... (1weight: Exercise 1; 2weights: all) ...
... (1weight: Exercise 1; 2weights: all) ...
How molecular orbital theory of metal ligand bonding in complexes
... Discuss π bonding in octahedral complexes on the basis of MOT. Discuss with example the lability and inertness of octahedral complexes according to VBT and CFT. What are electron transfer reactions? Explain the mechanism of one electron transfer reaction with suitable examples. What do understand by ...
... Discuss π bonding in octahedral complexes on the basis of MOT. Discuss with example the lability and inertness of octahedral complexes according to VBT and CFT. What are electron transfer reactions? Explain the mechanism of one electron transfer reaction with suitable examples. What do understand by ...
HS.Matter and Energy in Organisms and Ecosystems
... The process of photosynthesis converts light energy to stored chemical energy by converting carbon dioxide plus water into sugars plus released oxygen. (HS-LS1-5) The sugar molecules thus formed contain carbon, hydrogen, and oxygen: their hydrocarbon backbones are used to make amino acids and ot ...
... The process of photosynthesis converts light energy to stored chemical energy by converting carbon dioxide plus water into sugars plus released oxygen. (HS-LS1-5) The sugar molecules thus formed contain carbon, hydrogen, and oxygen: their hydrocarbon backbones are used to make amino acids and ot ...
Chemistry 20
... Explain that scientific knowledge is subject to change as new evidence becomes apparent and as laws and theories are tested and subsequently revised, reinforced or rejected. ...
... Explain that scientific knowledge is subject to change as new evidence becomes apparent and as laws and theories are tested and subsequently revised, reinforced or rejected. ...
Introduction to Computational Chemistry
... chemistry. Over the pas two decades computational methods have evolved into routine techniques that can be productively applied by every chemist in perfect analogy to, e.g. the use of an NMR spectrometer ...
... chemistry. Over the pas two decades computational methods have evolved into routine techniques that can be productively applied by every chemist in perfect analogy to, e.g. the use of an NMR spectrometer ...
111 Review Outline TRO
... When most reactions are performed, some of the reactants is usually present in excess of the amount needed. If the reaction goes to completion, then some of this excess reactant will be left-over. The limiting reactant is the reactant used-up completely and it "limits" the reaction. ...
... When most reactions are performed, some of the reactants is usually present in excess of the amount needed. If the reaction goes to completion, then some of this excess reactant will be left-over. The limiting reactant is the reactant used-up completely and it "limits" the reaction. ...
Chemistry 212 Name:
... Each halogen is obtained by oxidation of the halide ion to the halogen in a molten salt, except fluorine. None of the halogens is particularly abundant in nature, however all are easily accessible in concentrated forms rendering this point moot. All halogens have high electron affinities and ionizat ...
... Each halogen is obtained by oxidation of the halide ion to the halogen in a molten salt, except fluorine. None of the halogens is particularly abundant in nature, however all are easily accessible in concentrated forms rendering this point moot. All halogens have high electron affinities and ionizat ...
Sec. 12.3: Molecular Composition of Gases 1) Boyle`s Law: a
... 13) In 1808, Joseph Gay-Lussac made an important discovery: if the __________ and ______________ are kept constant, gases react in ____________ proportions that are __________ __________ __________. a) Gay-Lussac’s law of combining volumes: the law that states that the _____________ of gases involv ...
... 13) In 1808, Joseph Gay-Lussac made an important discovery: if the __________ and ______________ are kept constant, gases react in ____________ proportions that are __________ __________ __________. a) Gay-Lussac’s law of combining volumes: the law that states that the _____________ of gases involv ...
2. CHEMICAL ACTIVITY of the METALS 3. PATTERNS of the
... metal involves decomposition, which is endothermic and requires energy. Some compounds require more energy than others for decomposition. Copper and tin ores require little energy. A decent wood fire can “smelt” the metal from its ore. This why copper and bronze were used in ancient times. Iron ore ...
... metal involves decomposition, which is endothermic and requires energy. Some compounds require more energy than others for decomposition. Copper and tin ores require little energy. A decent wood fire can “smelt” the metal from its ore. This why copper and bronze were used in ancient times. Iron ore ...
Chapter 9
... System of Particles For a system of particles with com defined by: rcom = miri / M , Newton’s Second law : Fnet=M acom ...
... System of Particles For a system of particles with com defined by: rcom = miri / M , Newton’s Second law : Fnet=M acom ...
Nature of magnetism in double perovskite Ba2NaOsO6
... shells may resist breaking of lattice symmetry in cases where their lighter counterparts would succumb. Ba2NaOsO6 is a rare example of a Os7+ compound (d1: single occupation of the t2g orbits), also being ferromagnetic insulating (Tc ~ 7 K). Nevertheless, this system has the ideal cubic structure, i ...
... shells may resist breaking of lattice symmetry in cases where their lighter counterparts would succumb. Ba2NaOsO6 is a rare example of a Os7+ compound (d1: single occupation of the t2g orbits), also being ferromagnetic insulating (Tc ~ 7 K). Nevertheless, this system has the ideal cubic structure, i ...
Chapter 8 - Power Point Presentation
... surrounding the oxygen tend to arrange themselves as far from each other as possible in order to minimize repulsive forces. This results in a tetrahedral geometry in which the H-O-H bond angle would be 109.5°. However, the two lone pairs around the oxygen atom, have a greater space requirement, effe ...
... surrounding the oxygen tend to arrange themselves as far from each other as possible in order to minimize repulsive forces. This results in a tetrahedral geometry in which the H-O-H bond angle would be 109.5°. However, the two lone pairs around the oxygen atom, have a greater space requirement, effe ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.