Energy Level Models - Middle School Chemistry
... As the note on page 292 points out, there are other ways to model the electron energy levels of atoms. Some middle school texts show the electrons in pairs on an energy level. This pairing of electrons is intended to suggest information about the substructure within energy levels. This substructure ...
... As the note on page 292 points out, there are other ways to model the electron energy levels of atoms. Some middle school texts show the electrons in pairs on an energy level. This pairing of electrons is intended to suggest information about the substructure within energy levels. This substructure ...
Quantum Physics
... by none other than Albert Einstein. He was most famous for his Theory of Relativity, but he won his Nobel Prize for Physics due to this theory (photoelectric effect) which assumed that light may be considered as a stream of photons. The equation that summarises the photoelectric effect was named Ein ...
... by none other than Albert Einstein. He was most famous for his Theory of Relativity, but he won his Nobel Prize for Physics due to this theory (photoelectric effect) which assumed that light may be considered as a stream of photons. The equation that summarises the photoelectric effect was named Ein ...
Slide 1
... ii. Convert the amount of reagent one that was given into the number moles product that you could form if that reagent was completely consumed. ...
... ii. Convert the amount of reagent one that was given into the number moles product that you could form if that reagent was completely consumed. ...
Free electrons
... assume that there is some scattering mechanism. 3. Electron experiences a collision with a probability per unit time 1/ τ. The time τ − an average time between the two consecutive scattering events - known as, the collision time (relaxation time). The relaxation time τ is taken to be independent of ...
... assume that there is some scattering mechanism. 3. Electron experiences a collision with a probability per unit time 1/ τ. The time τ − an average time between the two consecutive scattering events - known as, the collision time (relaxation time). The relaxation time τ is taken to be independent of ...
Chapter 13 - "Water and Solutions"
... arrangement. (D) The water molecule has an angular arrangement called bent. If something were attached to the two lone pairs, it would be a tetrahedral arrangement. ...
... arrangement. (D) The water molecule has an angular arrangement called bent. If something were attached to the two lone pairs, it would be a tetrahedral arrangement. ...
Pretest Forces
... ______ 3. Any change in an object’s motion is called a. momentum. c. a force. b. an acceleration. d. velocity. ______ 4. Changes in an object’s motion are caused by a. a balanced force. c. an unbalanced force. b. an acceleration. d. opposing forces. ______ 5. The gravitational force between two obje ...
... ______ 3. Any change in an object’s motion is called a. momentum. c. a force. b. an acceleration. d. velocity. ______ 4. Changes in an object’s motion are caused by a. a balanced force. c. an unbalanced force. b. an acceleration. d. opposing forces. ______ 5. The gravitational force between two obje ...
Religion and the quantum world Transcript
... held, unless it exists in the mind of some observer, whether it is some finite spirit or the mind of God. Known as Idealism, this philosophical view has been unpopular in recent times, partly because science seemed to suggest that nothing exists except material particles, and that the mind is no mor ...
... held, unless it exists in the mind of some observer, whether it is some finite spirit or the mind of God. Known as Idealism, this philosophical view has been unpopular in recent times, partly because science seemed to suggest that nothing exists except material particles, and that the mind is no mor ...
Solid-state quantum computing using spectral holes M. S. Shahriar, P. R. Hemmer,
... minimum of decoherence. This method is analogous to a scheme proposed by Pellizzari et al. 关4兴 which uses adiabatic transfer to move quantum information coherently from one particle to another, then performs quantum logic by inducing single-particle Raman transitions. Consider a situation where each ...
... minimum of decoherence. This method is analogous to a scheme proposed by Pellizzari et al. 关4兴 which uses adiabatic transfer to move quantum information coherently from one particle to another, then performs quantum logic by inducing single-particle Raman transitions. Consider a situation where each ...
PPT
... Particles have position (and trajectories). If we measure position (e.g., which slit it went through) we have observed a particle property. That’s why the “which slit” measurement destroys the interference pattern. Note that particle and wave properties are incompatible. One can’t simultaneously mea ...
... Particles have position (and trajectories). If we measure position (e.g., which slit it went through) we have observed a particle property. That’s why the “which slit” measurement destroys the interference pattern. Note that particle and wave properties are incompatible. One can’t simultaneously mea ...
Chem 1A Final Exam – Fall 2005
... 8) Suppose you have a job in the Laney chemistry stockroom and your boss asks you to prepare 2.0000 L of a 0.250 M solution of ammonium fluoride. (Assume you have an analytical balance and 2.0000L, 1.0000L, 500.00mL, and 250.00mL volumetric flasks.) Describe in detail with the correct amounts ...
... 8) Suppose you have a job in the Laney chemistry stockroom and your boss asks you to prepare 2.0000 L of a 0.250 M solution of ammonium fluoride. (Assume you have an analytical balance and 2.0000L, 1.0000L, 500.00mL, and 250.00mL volumetric flasks.) Describe in detail with the correct amounts ...
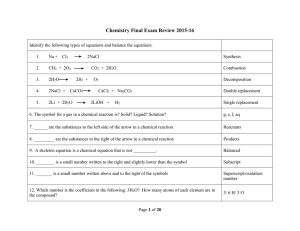
Type Of Chemical Reaction
... 42. The escape of high energy molecules from the liquid phase below the boiling point is called? ...
... 42. The escape of high energy molecules from the liquid phase below the boiling point is called? ...
Arrangement of Electrons in Atoms (Chapter 4) Notes
... II. The Photoelectric Effect (pg 93) – refers to the emission of electrons from a metal when light shines on the metal. The wave theory of light (early 1900) could not explain this phenomenon. The mystery of the photoelectric effect involved the frequency of the light striking the metal. For a give ...
... II. The Photoelectric Effect (pg 93) – refers to the emission of electrons from a metal when light shines on the metal. The wave theory of light (early 1900) could not explain this phenomenon. The mystery of the photoelectric effect involved the frequency of the light striking the metal. For a give ...
Transport Theory Breakdown of Onsager Symmetry in Neoclassical PFC/JA-82-31
... modifications of the boundary layer particle dynamics. This can cause a breakdown of the symmetry when turbulence is present. Whereas neoclassical theory can be viewed as a collisional scattering from one global collisionless orbit to another, in a turbulent medium the collisionless orbits are quite ...
... modifications of the boundary layer particle dynamics. This can cause a breakdown of the symmetry when turbulence is present. Whereas neoclassical theory can be viewed as a collisional scattering from one global collisionless orbit to another, in a turbulent medium the collisionless orbits are quite ...
Mass Relationships in Chemical Reactions
... It is process in which one or more pure substances are converted into one or more different pure substance. All chemical reactions involve a change in substances and a change in energy. Neither matter nor energy is created or destroyed in a chemical reaction, only changed. ...
... It is process in which one or more pure substances are converted into one or more different pure substance. All chemical reactions involve a change in substances and a change in energy. Neither matter nor energy is created or destroyed in a chemical reaction, only changed. ...
Dark Matter Gravity Waves Propel the EM Drive
... The paper presented a possible explanation of the EM Drive operation without violating the Newton’s third law, momentum conservation law, and the energy conservation law. A simple formula was derived for the force based on the assumption that the propellant that actually drives the EM Drive is the f ...
... The paper presented a possible explanation of the EM Drive operation without violating the Newton’s third law, momentum conservation law, and the energy conservation law. A simple formula was derived for the force based on the assumption that the propellant that actually drives the EM Drive is the f ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.