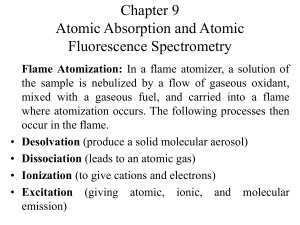
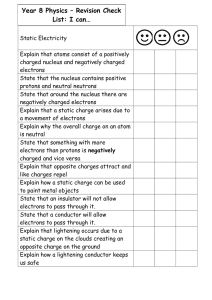
Document
... the positively charged nucleus is surrounded by a spherical electron cloud with equal and opposite charge. The dipole moment is zero. ...
... the positively charged nucleus is surrounded by a spherical electron cloud with equal and opposite charge. The dipole moment is zero. ...
Is the moon there when nobody looks?
... space A. If one adheres to this program, one can hardly consider the quantum-theoretical description as a complete representation of the physically real. If one tries to do so in spite of this, one has to assume that the physically real in B suffers a sudden change as a result of a measurement in A. ...
... space A. If one adheres to this program, one can hardly consider the quantum-theoretical description as a complete representation of the physically real. If one tries to do so in spite of this, one has to assume that the physically real in B suffers a sudden change as a result of a measurement in A. ...
Coherence conditions for groups of Rydberg atoms
... phase shifts needed to check the error of phase gates. Unless otherwise noted, atomic units will be used throughout this paper. 2. Theory In this section we will describe the techniques involved in the direct numerical solution to the many-atom wavefunction. We will also discuss how we solved for th ...
... phase shifts needed to check the error of phase gates. Unless otherwise noted, atomic units will be used throughout this paper. 2. Theory In this section we will describe the techniques involved in the direct numerical solution to the many-atom wavefunction. We will also discuss how we solved for th ...
Some results from the kinetic theory of gases
... We arrived at this result using a model that ignored collisions between molecules. The model is really just a convenience for calculation. It works for two reasons; the time spent during collisions is negligible compared to the time spent between collisions and, in a purely elastic exchange of momen ...
... We arrived at this result using a model that ignored collisions between molecules. The model is really just a convenience for calculation. It works for two reasons; the time spent during collisions is negligible compared to the time spent between collisions and, in a purely elastic exchange of momen ...
Oxidation-Reduction Reactions
... In a titration, a solution of accurately known concentration is added gradually to another solution of unknown concentration until the chemical reaction between the two solutions is ...
... In a titration, a solution of accurately known concentration is added gradually to another solution of unknown concentration until the chemical reaction between the two solutions is ...
Paper 3 - TheAllPapers
... possible. Every reasonable effort has been made by the publisher (UCLES) to trace copyright holders, but if any items requiring clearance have unwittingly been included, the publisher will be pleased to make amends at the earliest possible opportunity. University of Cambridge International Examinati ...
... possible. Every reasonable effort has been made by the publisher (UCLES) to trace copyright holders, but if any items requiring clearance have unwittingly been included, the publisher will be pleased to make amends at the earliest possible opportunity. University of Cambridge International Examinati ...
+ H 2 SO 4(aq) - Rothschild Science
... Conservation of Mass Law of Conservation of Mass In any physical or chemical reaction, mass is neither created nor destroyed; it is conserved! Reactants Products Same number of atoms on both sides of the equation! ...
... Conservation of Mass Law of Conservation of Mass In any physical or chemical reaction, mass is neither created nor destroyed; it is conserved! Reactants Products Same number of atoms on both sides of the equation! ...
Light interference from single atoms and their mirror images
... we recorded, simultaneously with the interference fringes, the ¯uorescence at 650 nm, which is transmitted through the mirror (see Fig. 1) and which is directly proportional to the population of the excited (P1/2) level of the ion. The result is shown in Fig. 3. The 650-nm ¯uorescence exhibits a cle ...
... we recorded, simultaneously with the interference fringes, the ¯uorescence at 650 nm, which is transmitted through the mirror (see Fig. 1) and which is directly proportional to the population of the excited (P1/2) level of the ion. The result is shown in Fig. 3. The 650-nm ¯uorescence exhibits a cle ...
Stage 2 Chemistry Intended Student Learning 2014
... The oxides of non-metals are acidic. Their acidic character can be displayed by reaction with hydroxide ions to produce an oxyanion and, in most cases, by reaction with water to ...
... The oxides of non-metals are acidic. Their acidic character can be displayed by reaction with hydroxide ions to produce an oxyanion and, in most cases, by reaction with water to ...
topic: chemical formula, chemical equations and stoichiometry
... It 1961 , Carbon atom as chosen as the standard element to be compare with. The relative atomic mass of carbon was fixed at 12.00 for carbon-12. Therefore, relative atomic mass was define as; “ the number of times the mass of one atom of an element is heavier than 1/12 times of one atom carbon-12 at ...
... It 1961 , Carbon atom as chosen as the standard element to be compare with. The relative atomic mass of carbon was fixed at 12.00 for carbon-12. Therefore, relative atomic mass was define as; “ the number of times the mass of one atom of an element is heavier than 1/12 times of one atom carbon-12 at ...
Chapter 3 The Statistical Theory of Thermodynamics 3.1 Macrostate
... A very simple example is a system in which each particle has just 2 energy levels, e.g., the ideal spin-1/2 paramagnet. At each of N sites we have an atom with spin S = 1/2. By quantum mechanics, the z-component has only 2 values: Sz = −1/2 for down-state (↓) and Sz = 1/2 for up-state (↑). If we app ...
... A very simple example is a system in which each particle has just 2 energy levels, e.g., the ideal spin-1/2 paramagnet. At each of N sites we have an atom with spin S = 1/2. By quantum mechanics, the z-component has only 2 values: Sz = −1/2 for down-state (↓) and Sz = 1/2 for up-state (↑). If we app ...
The Law of Conservation of Mechanical Energy
... The Law of Conservation of Mechanical Energy The law of conservation of mechanical energy states that “The sum of the kinetic energy and the potential energy in a system is constant if no resistant forces do work”. Mathematically, this law can be stated as: KE1 + PE1 = KE2 + PE2 = …….KEn + PEn In ot ...
... The Law of Conservation of Mechanical Energy The law of conservation of mechanical energy states that “The sum of the kinetic energy and the potential energy in a system is constant if no resistant forces do work”. Mathematically, this law can be stated as: KE1 + PE1 = KE2 + PE2 = …….KEn + PEn In ot ...
Quantum Mechanics Course essay Quantum mechanics Origins of
... – Electricity and magnetism progress from basic science to technological applications. ...
... – Electricity and magnetism progress from basic science to technological applications. ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.