Percent Composition and Molecular Formula Worksheet
... element is equal to the mass of each element in grams (show this during step #2) Step #2: Mass to mole Convert the mass of each element to moles of each element using the molar mass. Step #3: Divide by small Divide each of the mole quantities by the smallest number of moles. Often, this will res ...
... element is equal to the mass of each element in grams (show this during step #2) Step #2: Mass to mole Convert the mass of each element to moles of each element using the molar mass. Step #3: Divide by small Divide each of the mole quantities by the smallest number of moles. Often, this will res ...
Chemistry 211
... We have a balanced equation. It says “ 1 mole of methane + 2 moles of oxygen will produce 1 mole of carbon dioxide + 2 moles of water.” ...
... We have a balanced equation. It says “ 1 mole of methane + 2 moles of oxygen will produce 1 mole of carbon dioxide + 2 moles of water.” ...
Slide 1
... Plan: In part (a) we can make this prediction by determining the sign of ΔS° for the reaction and then using that information to analyze Equation 19.12. In part (b) we need to calculate ΔH° and ΔS° for the reaction by using the data in Appendix C. We can then use Equation 19.12 to calculate ΔG°. Sol ...
... Plan: In part (a) we can make this prediction by determining the sign of ΔS° for the reaction and then using that information to analyze Equation 19.12. In part (b) we need to calculate ΔH° and ΔS° for the reaction by using the data in Appendix C. We can then use Equation 19.12 to calculate ΔG°. Sol ...
19 BROWN Chemical Thermodynamics PPTSExercise
... Plan: In part (a) we can make this prediction by determining the sign of ΔS° for the reaction and then using that information to analyze Equation 19.12. In part (b) we need to calculate ΔH° and ΔS° for the reaction by using the data in Appendix C. We can then use Equation 19.12 to calculate ΔG°. Sol ...
... Plan: In part (a) we can make this prediction by determining the sign of ΔS° for the reaction and then using that information to analyze Equation 19.12. In part (b) we need to calculate ΔH° and ΔS° for the reaction by using the data in Appendix C. We can then use Equation 19.12 to calculate ΔG°. Sol ...
Stoichiometric relationships
... (a) The decomposition of copper carbonate (CuCO3) into copper oxide (CuO) and carbon dioxide ...
... (a) The decomposition of copper carbonate (CuCO3) into copper oxide (CuO) and carbon dioxide ...
CHEM_S1CourseReview_2011
... How do you identify the relative mass, relative charge, and location of the three smaller subatomic particles of an atom? What is the overall charge of an atom? What is the relationship between the subatomic particles and their charges, masses, and locations? What is an isotope? What is th ...
... How do you identify the relative mass, relative charge, and location of the three smaller subatomic particles of an atom? What is the overall charge of an atom? What is the relationship between the subatomic particles and their charges, masses, and locations? What is an isotope? What is th ...
Document
... Suppose you want to ‘whip’ a batch of hydrogen iodide, following the balanced chemical equation: ...
... Suppose you want to ‘whip’ a batch of hydrogen iodide, following the balanced chemical equation: ...
AP Chemistry: Total Notes Review
... change moles to grams 2. subtract the mass of C and H from the total mass to find the mass of O 3. convert grams to moles 4. divide by the smallest Take a Look: ...
... change moles to grams 2. subtract the mass of C and H from the total mass to find the mass of O 3. convert grams to moles 4. divide by the smallest Take a Look: ...
2. CHEMICAL ACTIVITY of the METALS 3. PATTERNS of the
... Hydrogen + Magnesium nitrate H2 + Mg(NO3)2 Hydrogen H2 ...
... Hydrogen + Magnesium nitrate H2 + Mg(NO3)2 Hydrogen H2 ...
ICSE Board Class X Chemistry Board Paper – 2015
... and carbon is a good reducing agent because of which zinc oxide gets easily reduced by carbon. Oxides of highly active metals like aluminium have great affinity towards oxygen and so cannot be reduced by carbon. (Note: Error in the question. Zinc oxide can be reduced to zinc metal by using carbon, b ...
... and carbon is a good reducing agent because of which zinc oxide gets easily reduced by carbon. Oxides of highly active metals like aluminium have great affinity towards oxygen and so cannot be reduced by carbon. (Note: Error in the question. Zinc oxide can be reduced to zinc metal by using carbon, b ...
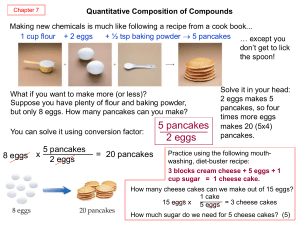
PowerPoint on Equivalent Ratios
... Sometimes you know where you want to go, but not what is needed to get there; this can happen in a ratio. I know that I want to use a certain amount of something, but do not know what that means for the other amount I use. https://www.youtube.com/watch?v=6MWTIcPLdg In this case I would cross mul ...
... Sometimes you know where you want to go, but not what is needed to get there; this can happen in a ratio. I know that I want to use a certain amount of something, but do not know what that means for the other amount I use. https://www.youtube.com/watch?v=6MWTIcPLdg In this case I would cross mul ...
Stoichiometery
... I have 1.6 mol of hydrogen. How much water can I make? A. 1.6 mol H2O B. 0.8 mol etc. C. 3.2 mol D. 4.8 mol E. None of the above ...
... I have 1.6 mol of hydrogen. How much water can I make? A. 1.6 mol H2O B. 0.8 mol etc. C. 3.2 mol D. 4.8 mol E. None of the above ...
Power Point for Equilibrium
... The Equilibrium Constant The Magnitude of Equilibrium Constants • The equilibrium constant, K, is the ratio of products to reactants. • Therefore, the larger K the more products are present at equilibrium. • Conversely, the smaller K the more reactants are present at equilibrium. • If K >> 1, then ...
... The Equilibrium Constant The Magnitude of Equilibrium Constants • The equilibrium constant, K, is the ratio of products to reactants. • Therefore, the larger K the more products are present at equilibrium. • Conversely, the smaller K the more reactants are present at equilibrium. • If K >> 1, then ...
CHAP 3.pmd - eVirtualGuru
... According to Dalton’s atomic theory, all matter, whether an element, a compound or a mixture is composed of small particles called atoms. The postulates of this theory may be stated as follows: (i) All matter is made of very tiny particles called atoms. (ii) Atoms are indivisible particles, which ca ...
... According to Dalton’s atomic theory, all matter, whether an element, a compound or a mixture is composed of small particles called atoms. The postulates of this theory may be stated as follows: (i) All matter is made of very tiny particles called atoms. (ii) Atoms are indivisible particles, which ca ...
Chapter 03
... A compound contains C, H, N. Combustion of 35.0mg of the compound produces 33.5mg CO2 and 41.1mg H2O. What is the empirical formula of the compound? Solution: 1. Determine C and H, the rest from 33.5mg is N. 2. Determine moles from masses. 3. Divide by smallest number of moles. Copyright©2000 by Hou ...
... A compound contains C, H, N. Combustion of 35.0mg of the compound produces 33.5mg CO2 and 41.1mg H2O. What is the empirical formula of the compound? Solution: 1. Determine C and H, the rest from 33.5mg is N. 2. Determine moles from masses. 3. Divide by smallest number of moles. Copyright©2000 by Hou ...
some basic concepts of chemistry
... Q. What are the limitations of law of definite proportions ? Solution :(1) The law is not applicable if an element exists in different isotopes which may be involved in the formation of the compound. For example, in the formation of the compound CO2, if C-12 isotope combines, the ratio of C : O is 1 ...
... Q. What are the limitations of law of definite proportions ? Solution :(1) The law is not applicable if an element exists in different isotopes which may be involved in the formation of the compound. For example, in the formation of the compound CO2, if C-12 isotope combines, the ratio of C : O is 1 ...
chapter i states of matter - myweb
... majority of chemical reactions are reversible only to some extent) and they always result in a change of a substance to a new one having different properties. An example of an irreversible chemical change is decomposition of water causing the molecules to break apart and form hydrogen and oxygen, tw ...
... majority of chemical reactions are reversible only to some extent) and they always result in a change of a substance to a new one having different properties. An example of an irreversible chemical change is decomposition of water causing the molecules to break apart and form hydrogen and oxygen, tw ...
Chemistry in Context: Chapter 3:The Chemistry of Global Warming
... • The mass of one Avogadro’s number (i.e. 1 mole) of molecular formula units of a chemical compound expressed in grams. • Molar mass is calculated by summing the atomic masses according to the molecular formula. ...
... • The mass of one Avogadro’s number (i.e. 1 mole) of molecular formula units of a chemical compound expressed in grams. • Molar mass is calculated by summing the atomic masses according to the molecular formula. ...
Mole Concept
... Isotopic masses cannot be obtained by summing the masses of the elementary particles (neutrons, protons, and electrons) from which the isotope is formed. This process would give masses slightly too large, since mass is lost when the neutrons and protons come together to form the nucleus. Atomic mass ...
... Isotopic masses cannot be obtained by summing the masses of the elementary particles (neutrons, protons, and electrons) from which the isotope is formed. This process would give masses slightly too large, since mass is lost when the neutrons and protons come together to form the nucleus. Atomic mass ...
Stoichiometry

Stoichiometry /ˌstɔɪkiˈɒmɨtri/ is the calculation of relative quantities of reactants and products in chemical reactions.Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of product can be empirically determined, then the amount of the other reactants can also be calculated.As seen in the image to the right, where the balanced equation is:CH4 + 2 O2 → CO2 + 2 H2O.Here, one molecule of methane reacts with two molecules of oxygen gas to yield one molecule of carbon dioxide and two molecules of water. Stoichiometry measures these quantitative relationships, and is used to determine the amount of products/reactants that are produced/needed in a given reaction. Describing the quantitative relationships among substances as they participate in chemical reactions is known as reaction stoichiometry. In the example above, reaction stoichiometry measures the relationship between the methane and oxygen as they react to form carbon dioxide and water.Because of the well known relationship of moles to atomic weights, the ratios that are arrived at by stoichiometry can be used to determine quantities by weight in a reaction described by a balanced equation. This is called composition stoichiometry.Gas stoichiometry deals with reactions involving gases, where the gases are at a known temperature, pressure, and volume and can be assumed to be ideal gases. For gases, the volume ratio is ideally the same by the ideal gas law, but the mass ratio of a single reaction has to be calculated from the molecular masses of the reactants and products. In practice, due to the existence of isotopes, molar masses are used instead when calculating the mass ratio.