Topic 1 - Coral Gables Senior High
... The term stoichiometry is derived from two Greek words – stoicheion for element and metron for measure. Stoichiometry describes the relationships between the amounts of reactants and products during chemical reactions. As it is known that matter is conserved during chemical change, stoichiometry is ...
... The term stoichiometry is derived from two Greek words – stoicheion for element and metron for measure. Stoichiometry describes the relationships between the amounts of reactants and products during chemical reactions. As it is known that matter is conserved during chemical change, stoichiometry is ...
PREPARATORY PROBLEMS (Theoretical)
... The natural tendency of any chemical reaction to proceed in a certain direction at constant temperature and pressure is determined by the sign of the Gibbs energy of the reaction, DG. This is the universal principle. If DG < 0, the reaction can proceed predominantly in the forward direction (a produ ...
... The natural tendency of any chemical reaction to proceed in a certain direction at constant temperature and pressure is determined by the sign of the Gibbs energy of the reaction, DG. This is the universal principle. If DG < 0, the reaction can proceed predominantly in the forward direction (a produ ...
PREPARATORY PROBLEMS (Theoretical)
... The natural tendency of any chemical reaction to proceed in a certain direction at constant temperature and pressure is determined by the sign of the Gibbs energy of the reaction, DG. This is the universal principle. If DG < 0, the reaction can proceed predominantly in the forward direction (a produ ...
... The natural tendency of any chemical reaction to proceed in a certain direction at constant temperature and pressure is determined by the sign of the Gibbs energy of the reaction, DG. This is the universal principle. If DG < 0, the reaction can proceed predominantly in the forward direction (a produ ...
PREPARATORY PROBLEMS
... The natural tendency of any chemical reaction to proceed in a certain direction at constant temperature and pressure is determined by the sign of the Gibbs energy of the reaction, DG. This is the universal principle. If DG < 0, the reaction can proceed predominantly in the forward direction (a produ ...
... The natural tendency of any chemical reaction to proceed in a certain direction at constant temperature and pressure is determined by the sign of the Gibbs energy of the reaction, DG. This is the universal principle. If DG < 0, the reaction can proceed predominantly in the forward direction (a produ ...
intermediate chemistry may 2011 marking scheme
... because of the great difference in electronegativities of the two elements, a H-bond exists between the partial positive H and the lone pair on the partially negatively charged O. (3) This electrostatic interaction is much stronger than the van der Waals forces in methane. (2) In methane, the simila ...
... because of the great difference in electronegativities of the two elements, a H-bond exists between the partial positive H and the lone pair on the partially negatively charged O. (3) This electrostatic interaction is much stronger than the van der Waals forces in methane. (2) In methane, the simila ...
Notes - Text
... equilibrium constant expression. Therefore, we anticipate that the amount of CO2 formed will not depend on the amounts of CaO and CaCO3 present. Note that although the concentrations of these species are not included in the equilibrium expression, they do participate in the reaction and must be pres ...
... equilibrium constant expression. Therefore, we anticipate that the amount of CO2 formed will not depend on the amounts of CaO and CaCO3 present. Note that although the concentrations of these species are not included in the equilibrium expression, they do participate in the reaction and must be pres ...
the properties and structure of matter
... its state (phase change), but does not change its chemical composition – E.g. Grinding, cutting ...
... its state (phase change), but does not change its chemical composition – E.g. Grinding, cutting ...
PIB - Unit 6 - Chemical Reactions - Student
... The substances that undergo a chemical reaction are the reactants. The new substances formed are the products. Special symbols are written after formulas in equations to show a substance’s state. The designations for solid, liquid, or gas, are (s), (l), and (g), respectively. A substance dissolv ...
... The substances that undergo a chemical reaction are the reactants. The new substances formed are the products. Special symbols are written after formulas in equations to show a substance’s state. The designations for solid, liquid, or gas, are (s), (l), and (g), respectively. A substance dissolv ...
Lab 1
... Primary substances, called elements, build all the materials about you. Some look similar, but others look unlike anything else. In this experiment, you will describe the physical properties of elements in a laboratory display and determine the location of elements on a blank periodic table. A. Phys ...
... Primary substances, called elements, build all the materials about you. Some look similar, but others look unlike anything else. In this experiment, you will describe the physical properties of elements in a laboratory display and determine the location of elements on a blank periodic table. A. Phys ...
Chemistry workbook
... Introduction to Balancing Chemical Reactions For each of the following count the number of moles of atoms of each element on each side of the yield( -->) sign, if they balance fine, if not, put in the correct numbers of moles required. Example : Rocket fuel is burned in a Saturn rocket. 2H2(g) + O2 ...
... Introduction to Balancing Chemical Reactions For each of the following count the number of moles of atoms of each element on each side of the yield( -->) sign, if they balance fine, if not, put in the correct numbers of moles required. Example : Rocket fuel is burned in a Saturn rocket. 2H2(g) + O2 ...
Molar mass
... simplest whole-number ratio of the elements in the formula. - Subscripts are used for these ratios. ...
... simplest whole-number ratio of the elements in the formula. - Subscripts are used for these ratios. ...
Chapter 3 Molecules, Compounds, and Chemical Equations
... Clicker question #3-9 A given hydrocarbon is converted completely to carbon dioxide and water, and equal numbers of moles of CO2 and H2O are produced. The hydrocarbon could be ...
... Clicker question #3-9 A given hydrocarbon is converted completely to carbon dioxide and water, and equal numbers of moles of CO2 and H2O are produced. The hydrocarbon could be ...
Chemistry Final Exam Study Guide
... ____ 73. According to the modern concept of the atom, which are located in the nucleus of an atom? a. electrons and protons c. neutrons and electrons b. protons only d. protons and neutrons ____ 74. An industrially important element contains 26 electrons and rusts in the presence of air and moisture ...
... ____ 73. According to the modern concept of the atom, which are located in the nucleus of an atom? a. electrons and protons c. neutrons and electrons b. protons only d. protons and neutrons ____ 74. An industrially important element contains 26 electrons and rusts in the presence of air and moisture ...
H o - CashmereChemistry
... 1. Write the data in the form of equations 2. Rewrite the equations to give the desired species on the correct side of the equation. If the reaction must be reversed (perhaps because we require a species to be a reactant and not a product) then the sign of the H must also be ...
... 1. Write the data in the form of equations 2. Rewrite the equations to give the desired species on the correct side of the equation. If the reaction must be reversed (perhaps because we require a species to be a reactant and not a product) then the sign of the H must also be ...
Microbial Biogeochemistry
... Bacteria that are able to use the most energetic reactions in their surrounding environment will dominate that microenvironment. Transport combined with the microbial sources and sinks will determine the resulting chemical gradients. Chemical gradients can be transient as substrates are exhausted or ...
... Bacteria that are able to use the most energetic reactions in their surrounding environment will dominate that microenvironment. Transport combined with the microbial sources and sinks will determine the resulting chemical gradients. Chemical gradients can be transient as substrates are exhausted or ...
Experimental and Simulation Results for the Removal of H2S from
... substitutable for fossil fuels. This gas produced from the wastewater treatment by degradation of organic matter under anaerobic conditions is mainly composed of methane and carbon dioxide. To be used as a renewable fuel, biogas, whose energy comes only from methane, must be purified from carbon dio ...
... substitutable for fossil fuels. This gas produced from the wastewater treatment by degradation of organic matter under anaerobic conditions is mainly composed of methane and carbon dioxide. To be used as a renewable fuel, biogas, whose energy comes only from methane, must be purified from carbon dio ...
Unit 5 Student Packet
... change from 25.246C to 26.386C. If the heat capacity of the calorimeter was 7.15 kJ/C, calculate H comb for octane. 3. Calcium oxide (lime) reacts with water to give calcium hydroxide. A 5.40 g sample of calcium oxide was added to 500. mL of water in a calorimeter of heat capacity 350. J/C. The ...
... change from 25.246C to 26.386C. If the heat capacity of the calorimeter was 7.15 kJ/C, calculate H comb for octane. 3. Calcium oxide (lime) reacts with water to give calcium hydroxide. A 5.40 g sample of calcium oxide was added to 500. mL of water in a calorimeter of heat capacity 350. J/C. The ...
The Wizard Test Maker
... An experiment is set up to determine the molecular mass of a water-soluble, nonvolatile, non-electrolyte. The equipment listed above is availeable to use. No other equipment is available. (a) Briefly list the steps needed to carry out this experiment. (b) What experimental data needs to be collected ...
... An experiment is set up to determine the molecular mass of a water-soluble, nonvolatile, non-electrolyte. The equipment listed above is availeable to use. No other equipment is available. (a) Briefly list the steps needed to carry out this experiment. (b) What experimental data needs to be collected ...
Summary - Clydebank High School
... molecules and also between the atoms of ............................. gases (group 8). .................... der ................... forces are much .................................. than all other types of bonding. 5. Van der Waals forces are caused by small electrostatic attractions between ...... ...
... molecules and also between the atoms of ............................. gases (group 8). .................... der ................... forces are much .................................. than all other types of bonding. 5. Van der Waals forces are caused by small electrostatic attractions between ...... ...
College Chemistry 1 Note Guide(free download)
... significantly from properly using the note guide. It is such an effective aid that students enrolling in the lecture course at the college frequently purchase the note guide to assist their note taking and learning. The reference made to a syllabus during the first lesson refers to the syllabus for ...
... significantly from properly using the note guide. It is such an effective aid that students enrolling in the lecture course at the college frequently purchase the note guide to assist their note taking and learning. The reference made to a syllabus during the first lesson refers to the syllabus for ...
Chemistry Bridging Work
... a) How many moles of water are needed to react with 0.03 moles of carbon dioxide? b) How many moles of glucose can you make from 0.03 moles of carbon dioxide? c) How many moles of oxygen can you make from 0.03 moles of carbon dioxide? ...
... a) How many moles of water are needed to react with 0.03 moles of carbon dioxide? b) How many moles of glucose can you make from 0.03 moles of carbon dioxide? c) How many moles of oxygen can you make from 0.03 moles of carbon dioxide? ...
Chemical properties Chemical properties can be recognized only
... Chemical properties Chemical properties can be recognized only when substances react or do not react chemically with one another, that is, when they undergo a change in composition. The following chemical properties can be used to help identify a substance: Ability to burn The ability to burn involv ...
... Chemical properties Chemical properties can be recognized only when substances react or do not react chemically with one another, that is, when they undergo a change in composition. The following chemical properties can be used to help identify a substance: Ability to burn The ability to burn involv ...
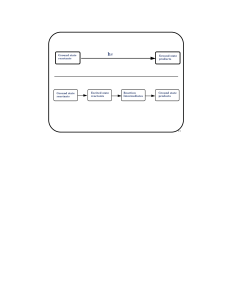
Ground state reactants Ground state products Ground state
... Energy levels for molecular oxygen. Excited triplet states have not been included because they are much higher in energy. The 1∆g state is the one normally refereed to as singlet oxygen. ...
... Energy levels for molecular oxygen. Excited triplet states have not been included because they are much higher in energy. The 1∆g state is the one normally refereed to as singlet oxygen. ...
Stoichiometry

Stoichiometry /ˌstɔɪkiˈɒmɨtri/ is the calculation of relative quantities of reactants and products in chemical reactions.Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of product can be empirically determined, then the amount of the other reactants can also be calculated.As seen in the image to the right, where the balanced equation is:CH4 + 2 O2 → CO2 + 2 H2O.Here, one molecule of methane reacts with two molecules of oxygen gas to yield one molecule of carbon dioxide and two molecules of water. Stoichiometry measures these quantitative relationships, and is used to determine the amount of products/reactants that are produced/needed in a given reaction. Describing the quantitative relationships among substances as they participate in chemical reactions is known as reaction stoichiometry. In the example above, reaction stoichiometry measures the relationship between the methane and oxygen as they react to form carbon dioxide and water.Because of the well known relationship of moles to atomic weights, the ratios that are arrived at by stoichiometry can be used to determine quantities by weight in a reaction described by a balanced equation. This is called composition stoichiometry.Gas stoichiometry deals with reactions involving gases, where the gases are at a known temperature, pressure, and volume and can be assumed to be ideal gases. For gases, the volume ratio is ideally the same by the ideal gas law, but the mass ratio of a single reaction has to be calculated from the molecular masses of the reactants and products. In practice, due to the existence of isotopes, molar masses are used instead when calculating the mass ratio.