2.10 Basic Nuclear Chemistry
... 1. Nucleons exist in different energy levels/shells within the nucleus, just like electrons in the cloud. 2. Even numbers tend to be more stable than odd numbers of nucleons. B. As the number of protons in a nucleus increases, the stability of the nucleus decreases 1. This is because the positive re ...
... 1. Nucleons exist in different energy levels/shells within the nucleus, just like electrons in the cloud. 2. Even numbers tend to be more stable than odd numbers of nucleons. B. As the number of protons in a nucleus increases, the stability of the nucleus decreases 1. This is because the positive re ...
Chapter 4 Assignment Answers
... b. Thomson observed the same cathode rays with all of the different metals that he used. 39. Two electrons should repel each other. 40. The mass of a neutron is equal to the mass of a proton: 1 amu. However a proton is (+) charged and a neutron is neutral. 41. When an atom loses electrons, there are ...
... b. Thomson observed the same cathode rays with all of the different metals that he used. 39. Two electrons should repel each other. 40. The mass of a neutron is equal to the mass of a proton: 1 amu. However a proton is (+) charged and a neutron is neutral. 41. When an atom loses electrons, there are ...
Section III: A World of Particles
... more complex, that facilitates understanding certain aspects of a real object or process. Atoms: The smallest unit of an element that retains the chemical properties of that element. ...
... more complex, that facilitates understanding certain aspects of a real object or process. Atoms: The smallest unit of an element that retains the chemical properties of that element. ...
25.3 section summary
... Nuclear fission occurs when fissionable isotopes are bombarded with neutrons. The fissionable atom breaks into two fragments of about the same size, and in the process they release more neutrons and energy. Neutron moderation is the process that reduces the speed of neutrons. Sometimes water is used ...
... Nuclear fission occurs when fissionable isotopes are bombarded with neutrons. The fissionable atom breaks into two fragments of about the same size, and in the process they release more neutrons and energy. Neutron moderation is the process that reduces the speed of neutrons. Sometimes water is used ...
Biology Fall Semester Test 1 Study Guide
... Cellular respiration and photosynthesis are responsible for recycling which two nutrients? A well-tested explanation that unifies a broad range of observations is a(an) The process by which organisms keep their internal conditions fairly constant is called In the metric system, the basic unit of len ...
... Cellular respiration and photosynthesis are responsible for recycling which two nutrients? A well-tested explanation that unifies a broad range of observations is a(an) The process by which organisms keep their internal conditions fairly constant is called In the metric system, the basic unit of len ...
radioactivity-ppt
... unstable atomic nucleus loses energy by emitting radiation in the form of particles or electromagnetic waves. There are numerous types of radioactive decay. The ...
... unstable atomic nucleus loses energy by emitting radiation in the form of particles or electromagnetic waves. There are numerous types of radioactive decay. The ...
Radioactivity overview
... 1∙ seconds. Electron and proton have got corresponding antiparticles – positron and anti-proton with lifetimes less s. Except these particles, should note another atomic particle - photon (γ), which does not have rest mass and with unlimited lifetime. Atomic nuclei are able to emit more than 25 elem ...
... 1∙ seconds. Electron and proton have got corresponding antiparticles – positron and anti-proton with lifetimes less s. Except these particles, should note another atomic particle - photon (γ), which does not have rest mass and with unlimited lifetime. Atomic nuclei are able to emit more than 25 elem ...
Chemistry 2.2: Protons, Neutrons and Electrons Protons, neutrons
... subatomic particles found in an atom. All atoms have three basic subatomic particles: p_______, n________, and ...
... subatomic particles found in an atom. All atoms have three basic subatomic particles: p_______, n________, and ...
Nuclear Physics SL - Hockerill Students
... In Einstein’s equation, 1kg of mass is equivalent to 9x1016J of energy. Since mass and energy are equivalent it is sometimes useful to work in units that avoid having to do repeated multiplications by the speed of light. ...
... In Einstein’s equation, 1kg of mass is equivalent to 9x1016J of energy. Since mass and energy are equivalent it is sometimes useful to work in units that avoid having to do repeated multiplications by the speed of light. ...
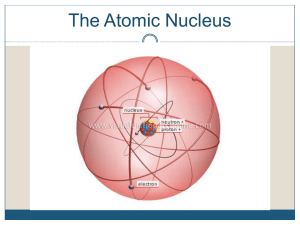
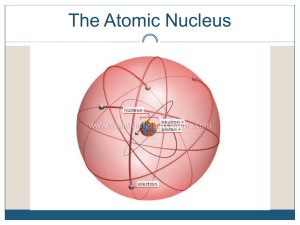
Atomic nucleus
The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The atomic nucleus was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.The diameter of the nucleus is in the range of 6985175000000000000♠1.75 fm (6985175000000000000♠1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 6986150000000000000♠15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.