
Unit 3 - Section 5.3 2011 Atoms and Molecules DVD
... Protons have a positive charge and electrons have a negative charge, and just like a magnet, these opposite charges attract. That is, protons attract electrons. The negative electrons are attracted to the atom’s nucleus because the nucleus contains positive protons. The attractive force is called EL ...
... Protons have a positive charge and electrons have a negative charge, and just like a magnet, these opposite charges attract. That is, protons attract electrons. The negative electrons are attracted to the atom’s nucleus because the nucleus contains positive protons. The attractive force is called EL ...
Grade 10 Applied Science – Unit Chemistry
... Protons have a positive charge and electrons have a negative charge, and just like a magnet, these opposite charges attract. That is, protons attract electrons. The negative electrons are attracted to the atom’s nucleus because the nucleus contains positive protons. The attractive force is called EL ...
... Protons have a positive charge and electrons have a negative charge, and just like a magnet, these opposite charges attract. That is, protons attract electrons. The negative electrons are attracted to the atom’s nucleus because the nucleus contains positive protons. The attractive force is called EL ...
Atoms and Elements
... Building Blocks of Matter Atom- A basic unit of matter consisting of a dense central nucleus surrounded by a cloud of negatively charged electrons. • Element- A pure chemical substance composed of one type of atom. • Periodic Table of the Elements- An arrangement of elements in columns based on a s ...
... Building Blocks of Matter Atom- A basic unit of matter consisting of a dense central nucleus surrounded by a cloud of negatively charged electrons. • Element- A pure chemical substance composed of one type of atom. • Periodic Table of the Elements- An arrangement of elements in columns based on a s ...
Nuclear Chemistry - Northwest ISD Moodle
... • PROTONS give the atom its identity • Held together by a very strong nuclear force o One of the four fundamental forces in our universe o Incredibly powerful o Releasing nuclear force results in a nuclear reaction ...
... • PROTONS give the atom its identity • Held together by a very strong nuclear force o One of the four fundamental forces in our universe o Incredibly powerful o Releasing nuclear force results in a nuclear reaction ...
Atomic Structure | Topic Notes
... Rutherford’s Gold Foil • Geiger & Marsden • fire α-particles at thin gold foil • large no. not deflected - essentially empty space • many deflected at small angles • some deflected at large angles - passed close to positive charge • few rebounded - collided directly with a small, dense nucleus of po ...
... Rutherford’s Gold Foil • Geiger & Marsden • fire α-particles at thin gold foil • large no. not deflected - essentially empty space • many deflected at small angles • some deflected at large angles - passed close to positive charge • few rebounded - collided directly with a small, dense nucleus of po ...
Document
... An industrially important element contains 26 electrons and rusts in the presence of air and moisture. Identify the element. ...
... An industrially important element contains 26 electrons and rusts in the presence of air and moisture. Identify the element. ...
o Atomic Number = Protons = Electrons o Mass – Atomic Number
... http://chemistry.tutorvista.com/nuclear-chemistry/properties-of-subatomic-particles.html ...
... http://chemistry.tutorvista.com/nuclear-chemistry/properties-of-subatomic-particles.html ...
Physics 535 lectures notes: 1 * Sep 4th 2007
... a) Why did we observe the nuclear masses that we see? For instance, why was Helium four times more massive than hydrogen rather than twice? b) How did you bind the positively charged particles together when they should have a strong electromagnetic repulsion? c) How to explain other “radiation” emit ...
... a) Why did we observe the nuclear masses that we see? For instance, why was Helium four times more massive than hydrogen rather than twice? b) How did you bind the positively charged particles together when they should have a strong electromagnetic repulsion? c) How to explain other “radiation” emit ...
Atomic History and Structure Atomic Timeline Dalton (Indivisible
... Atomic Radius is a measure of the distance from the edge to the center an atom – the radius of the “spherical” atom. Different elements have different atomic radii due to electrostatic forces in the atom. Nuclear Charge is is the positive attractive charge the nucleus exhibits on electrons and incre ...
... Atomic Radius is a measure of the distance from the edge to the center an atom – the radius of the “spherical” atom. Different elements have different atomic radii due to electrostatic forces in the atom. Nuclear Charge is is the positive attractive charge the nucleus exhibits on electrons and incre ...
Chapter 5 Review Answer Key
... Thompson put gas into a glass tube at a near-vacuum and put a charge through it, causing a beam of light. When an electromagnet was placed near the tube, the beam was deflected away from the negative and towards the positive. The results were the same for all gases he used, thus he proved that since ...
... Thompson put gas into a glass tube at a near-vacuum and put a charge through it, causing a beam of light. When an electromagnet was placed near the tube, the beam was deflected away from the negative and towards the positive. The results were the same for all gases he used, thus he proved that since ...
Dominoes - Learning on the Loop
... The force that binds neutrons and protons together in the nucleus ...
... The force that binds neutrons and protons together in the nucleus ...
Nuclear Chemistry
... There are certain numbers of protons and neutrons that produce very stable nuclei. These numbers are referred to magic numbers and are 2, 8, 20, 28, 50, 82, and ...
... There are certain numbers of protons and neutrons that produce very stable nuclei. These numbers are referred to magic numbers and are 2, 8, 20, 28, 50, 82, and ...
Notes – Atomic Structure
... - Some types of atoms have nuclei that are not stable. That is the nucleus is unable to hold itself together and as such is shoots out particles or energy as it falls apart. Atoms that do this are said to be radioactive or undergo radioactive decay. - Further investigation into radioactive decay dis ...
... - Some types of atoms have nuclei that are not stable. That is the nucleus is unable to hold itself together and as such is shoots out particles or energy as it falls apart. Atoms that do this are said to be radioactive or undergo radioactive decay. - Further investigation into radioactive decay dis ...
Nuclear Physics and Radioactivity
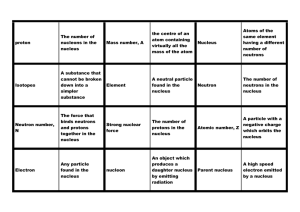
... atomic mass number (A) - the number of protons and neutrons in the nucleus of an atom. atomic mass unit - the unit of mass equal to 1/12 the mass of a carbon-12 nucleus; the atomic mass rounded to the nearest whole number is called the mass number. atomic number (Z) - the number of protons in the nu ...
... atomic mass number (A) - the number of protons and neutrons in the nucleus of an atom. atomic mass unit - the unit of mass equal to 1/12 the mass of a carbon-12 nucleus; the atomic mass rounded to the nearest whole number is called the mass number. atomic number (Z) - the number of protons in the nu ...
Chapter 6.1 Q1 (a) The mass of the nucleus is approximately 56 u
... Q11 A typical nucleus has a radius that is a few times larger than the range of the nuclear force. For example the radius of the nucleus of tin ( Sn with mass number A = 119 ) is R = 1.2 ! 1191/ 3 ! 10 "15 = 5.9 ! 10 "15 m , i.e. about 6 times the range of the nuclear force. A proton within the nucl ...
... Q11 A typical nucleus has a radius that is a few times larger than the range of the nuclear force. For example the radius of the nucleus of tin ( Sn with mass number A = 119 ) is R = 1.2 ! 1191/ 3 ! 10 "15 = 5.9 ! 10 "15 m , i.e. about 6 times the range of the nuclear force. A proton within the nucl ...
Chapter 4 Key Terms - Lower Cape May Regional School District
... atomic number - the number of protons in the nucleus of an atom average atomic mass - the weighted average of the masses of all naturally occurring isotopes of an element mass number - the total number of protons and neutrons in the nucleus of an atom isotopes - any atoms having the same number of p ...
... atomic number - the number of protons in the nucleus of an atom average atomic mass - the weighted average of the masses of all naturally occurring isotopes of an element mass number - the total number of protons and neutrons in the nucleus of an atom isotopes - any atoms having the same number of p ...
Atomic nucleus
The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The atomic nucleus was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.The diameter of the nucleus is in the range of 6985175000000000000♠1.75 fm (6985175000000000000♠1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 6986150000000000000♠15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.














![LC Atomic Structure [PDF Document]](http://s1.studyres.com/store/data/003605537_1-38ee6ce36c748f8bb00c7b0a30a77f99-300x300.png)






