Document
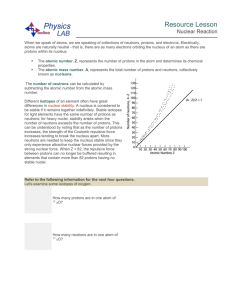
... During beta decay, the daughter nucleus has the same number of nucleons as the parent, but the atomic number is one less In addition, an electron (positron) was observed The emission of the electron is from the nucleus ...
... During beta decay, the daughter nucleus has the same number of nucleons as the parent, but the atomic number is one less In addition, an electron (positron) was observed The emission of the electron is from the nucleus ...
SURA Meeting: Section 6 – Density Functional Approach
... intrinsic scientific interest, such data will play an important role in nuclear astrophysics, including the understanding of the structure and properties of neutron stars. In such dense systems one also expects hyperons to play a significant role and facilities aimed at measuring the properties of n ...
... intrinsic scientific interest, such data will play an important role in nuclear astrophysics, including the understanding of the structure and properties of neutron stars. In such dense systems one also expects hyperons to play a significant role and facilities aimed at measuring the properties of n ...
Document
... 39) Which property of aluminum makes it a suitable metal for soft drink cans? a) It has good electrical conductivity b) It can be hammered into a thin sheet (malleability) c) It can be drawn into long wires (ductility) ...
... 39) Which property of aluminum makes it a suitable metal for soft drink cans? a) It has good electrical conductivity b) It can be hammered into a thin sheet (malleability) c) It can be drawn into long wires (ductility) ...
Atomic Nucleus (Eng) - George P. Shpenkov
... where the majority of the mass of the atom, as he assumed, was concentrated. Other possible options to explain the phenomenon of backscattering he did not consider. Having taken as a result the nuclear model of the atom for a basis, Rutherford and his followers were forced to admit that in this case ...
... where the majority of the mass of the atom, as he assumed, was concentrated. Other possible options to explain the phenomenon of backscattering he did not consider. Having taken as a result the nuclear model of the atom for a basis, Rutherford and his followers were forced to admit that in this case ...
Nuclear Radiation and Decay File
... • Because the atom now has one more proton, it becomes the element with an atomic number one greater than that of the original element. ...
... • Because the atom now has one more proton, it becomes the element with an atomic number one greater than that of the original element. ...
nuclear test 2006
... Carbon Dating is a technique used to determine the age of organic material. The activity (rate of decay) of 14C atoms in the sample is measured. 14C has a half life of 5730 years. a) Explain how the activity of 14C in an organic sample can be used to determine its age. ...
... Carbon Dating is a technique used to determine the age of organic material. The activity (rate of decay) of 14C atoms in the sample is measured. 14C has a half life of 5730 years. a) Explain how the activity of 14C in an organic sample can be used to determine its age. ...
Distinguishing Between Atoms PPT
... The following is shorthand for writing the # of protons and neutrons, you don’t need to write the # of electrons because they are the same as the # of protons The large "X" represents where you will find the atom's elemental symbol. The mass number, which is given the symbol "A", is located in the ...
... The following is shorthand for writing the # of protons and neutrons, you don’t need to write the # of electrons because they are the same as the # of protons The large "X" represents where you will find the atom's elemental symbol. The mass number, which is given the symbol "A", is located in the ...
Study Guide 1st Semester
... 37. What is the electron configuration for the following elements? (short hand or long hand is fine)? a) Gallium d) Magnesium b) Zirconium e) Iodine c) Californium f) Copper 38. What did Dalton, Thomson, Rutherford, Bohr, and Schrodinger have to say about the structure of the atoms (description and ...
... 37. What is the electron configuration for the following elements? (short hand or long hand is fine)? a) Gallium d) Magnesium b) Zirconium e) Iodine c) Californium f) Copper 38. What did Dalton, Thomson, Rutherford, Bohr, and Schrodinger have to say about the structure of the atoms (description and ...
Electrons Circulating a Nucleus
... •The word “quantum” (Latin, “how much”) in quantum mechanics refers to a discrete unit that quantum theory assigns to certain physical quantities, such as the energy of an atom at rest (see Figure 1, at right). The discovery that waves have discrete energy packets (called quanta) that behave in a ma ...
... •The word “quantum” (Latin, “how much”) in quantum mechanics refers to a discrete unit that quantum theory assigns to certain physical quantities, such as the energy of an atom at rest (see Figure 1, at right). The discovery that waves have discrete energy packets (called quanta) that behave in a ma ...
Nuclear Medicine
... There are 82 stable elements and about 275 stable isotopes of these elements. When a combination of neutrons and protons, which does not already exist in nature, is produced artificially, the atom will be unstable and is called a radioactive isotope or radioisotope. There are also a number of unstab ...
... There are 82 stable elements and about 275 stable isotopes of these elements. When a combination of neutrons and protons, which does not already exist in nature, is produced artificially, the atom will be unstable and is called a radioactive isotope or radioisotope. There are also a number of unstab ...
Year 11 Chemistry Balancing Equations
... water, and the soluble salt calcium nitrate Ca(NO3)2 c When dilute sodium sulfate Na2SO4 solution is added to dilute barium nitrate Ba(NO3)2 solution, barium sulfate BaSO4 precipitates, leaving sodium nitrate NaNO3 in solution. d Dilute sodium hydroxide is added to dilute sulfuric acid H2SO4, produc ...
... water, and the soluble salt calcium nitrate Ca(NO3)2 c When dilute sodium sulfate Na2SO4 solution is added to dilute barium nitrate Ba(NO3)2 solution, barium sulfate BaSO4 precipitates, leaving sodium nitrate NaNO3 in solution. d Dilute sodium hydroxide is added to dilute sulfuric acid H2SO4, produc ...
The Interaction of Mechanical Force and Electric Charge in Physical
... distinctive property: their electric charge. It must be more complicated than simply opposite electric charges. Electrons are not thought to stick to the surface of protons, as electric charge might lead us to expect, but continue in a sort of perpetual motion. In the case of the hydrogen atom, reas ...
... distinctive property: their electric charge. It must be more complicated than simply opposite electric charges. Electrons are not thought to stick to the surface of protons, as electric charge might lead us to expect, but continue in a sort of perpetual motion. In the case of the hydrogen atom, reas ...
Flashback 2
... Two positively charged particles at rest exert a force of 4.6 103 N on one another. The charge of the first particle is 6.0 10-5 C and the charge of the second particle is 2.0 10-4 C. What is the distance between the two charged particles? ...
... Two positively charged particles at rest exert a force of 4.6 103 N on one another. The charge of the first particle is 6.0 10-5 C and the charge of the second particle is 2.0 10-4 C. What is the distance between the two charged particles? ...
Atomic nucleus
The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The atomic nucleus was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.The diameter of the nucleus is in the range of 6985175000000000000♠1.75 fm (6985175000000000000♠1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 6986150000000000000♠15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.