Physical Science: Ch. 10 - Pleasant Hill Elementary School
... They’re still cats, just like an isotope of carbon is still carbon. They just happen to have a different number of digits, like C-14 happens to have 8 neutrons. ...
... They’re still cats, just like an isotope of carbon is still carbon. They just happen to have a different number of digits, like C-14 happens to have 8 neutrons. ...
chapter 21 blm answer key
... When the fission process is critical, one neutron from each fission event causes one more fission event. At the critical level, the reaction is sustained at a constant rate. When the process is subcritical, fewer than one neutron from each fission event causes a further fission event. The reaction s ...
... When the fission process is critical, one neutron from each fission event causes one more fission event. At the critical level, the reaction is sustained at a constant rate. When the process is subcritical, fewer than one neutron from each fission event causes a further fission event. The reaction s ...
Document
... 5. Know the locations and label the names for the following groups: 1, 2, 3-12, 13, 14, ...
... 5. Know the locations and label the names for the following groups: 1, 2, 3-12, 13, 14, ...
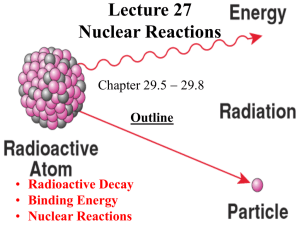
5.7 Nuclear Radiation
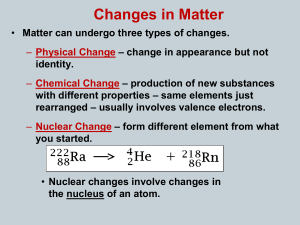
... – The process continues until unstable isotopes of one element are changed, or transformed, into stable isotopes of a different element. – These stable isotopes are not radioactive. – Nuclear radiation is emitted during radioactive decay. – There are three main types of nuclear radiation: alpha rad ...
... – The process continues until unstable isotopes of one element are changed, or transformed, into stable isotopes of a different element. – These stable isotopes are not radioactive. – Nuclear radiation is emitted during radioactive decay. – There are three main types of nuclear radiation: alpha rad ...
Primary electrons make random elastic and inelastic collision either
... Signals such as secondary electrons (SE) and Auger electrons come from only a very tiny portion of the total interaction volume, since they lack the energy to travel large distances… Only these SE electrons that originated within a few nanometer of the surface are able to escape from sample…. SE has ...
... Signals such as secondary electrons (SE) and Auger electrons come from only a very tiny portion of the total interaction volume, since they lack the energy to travel large distances… Only these SE electrons that originated within a few nanometer of the surface are able to escape from sample…. SE has ...
particle - Uplift North Hills
... The stable nuclides of the lighter elements have approximately equal numbers of protons and neutrons? However, as Z increases the `stability line' curves upwards. Heavier nuclei need more and more neutrons to be stable. Can we explain why? ● It is the strong nuclear force that holds the nucleons to ...
... The stable nuclides of the lighter elements have approximately equal numbers of protons and neutrons? However, as Z increases the `stability line' curves upwards. Heavier nuclei need more and more neutrons to be stable. Can we explain why? ● It is the strong nuclear force that holds the nucleons to ...
Chapter 11 Review Worksheet
... 7. What is the symbol for the lowest-energy hydrogen orbital? 1S 8. Give the symbols for each of the orbitals that constitute the third and fourth principle energy levels of hydrogen. Third – s, p, d Fourth – s, p, d, f 9. Describe electron spin. Electrons are spinning on their axis and rotating ar ...
... 7. What is the symbol for the lowest-energy hydrogen orbital? 1S 8. Give the symbols for each of the orbitals that constitute the third and fourth principle energy levels of hydrogen. Third – s, p, d Fourth – s, p, d, f 9. Describe electron spin. Electrons are spinning on their axis and rotating ar ...
Chapter 15 Review - korman
... 4. These elements are neatly arranged on the ___________________________by their _____________________ or number of ____________ & ______________ (if neutral) in each element’s unique atom. 5. To determine the number of _______________ in an average atom of an element you will need to subtract the _ ...
... 4. These elements are neatly arranged on the ___________________________by their _____________________ or number of ____________ & ______________ (if neutral) in each element’s unique atom. 5. To determine the number of _______________ in an average atom of an element you will need to subtract the _ ...
Chapter 3 section 2 review and key
... 6. A cathode ray produced in a gas-filled tube is deflected by a magnetic field. A wire carrying an electric current can be pulled by a magnetic field. A cathode ray is deflected away from a negatively charged object. What property of the cathode ray is shown by these phenomena? The particles that c ...
... 6. A cathode ray produced in a gas-filled tube is deflected by a magnetic field. A wire carrying an electric current can be pulled by a magnetic field. A cathode ray is deflected away from a negatively charged object. What property of the cathode ray is shown by these phenomena? The particles that c ...
ERC-focus (English)
... plays a role in the so-called weak decay of particles through the exchange of yet another set of force particles, which have large masses coming from the Higgs field. The recent likely discovery of the excitation of this field is an important step in our quest what is beyond the so-called Standard M ...
... plays a role in the so-called weak decay of particles through the exchange of yet another set of force particles, which have large masses coming from the Higgs field. The recent likely discovery of the excitation of this field is an important step in our quest what is beyond the so-called Standard M ...
Reakcje jądrowe
... light is very high and equals about 3x108 m/s in a vacuum. We take the same value in the air. We can increase magnetic field or decrease frequency of potential difference applying to daunts, or both, to avoid increases of the particle’s mass. We have a phase-tron, cyclotron, or phase-cyclotron, resp ...
... light is very high and equals about 3x108 m/s in a vacuum. We take the same value in the air. We can increase magnetic field or decrease frequency of potential difference applying to daunts, or both, to avoid increases of the particle’s mass. We have a phase-tron, cyclotron, or phase-cyclotron, resp ...
Class Note Packet: Atomic Theory Main Idea Details The Structure of
... _______________________expected the paths of the alpha particles to be only slightly altered by a collision with the electron. Although most of the alpha particles went through the gold foil a few of them ________________ back, some at large angles. That atoms are mostly _____________ space. Almost ...
... _______________________expected the paths of the alpha particles to be only slightly altered by a collision with the electron. Although most of the alpha particles went through the gold foil a few of them ________________ back, some at large angles. That atoms are mostly _____________ space. Almost ...
Semester Exam Review Guide
... 24. Plasmas include all of the following except: a. ionized gases b. lava c. lightning d. stars 26. If the mass of a steel bolt is 4.0 grams and its volume is 2 milliliters, what is the bolt’s density? a. 2 ml / g b. 2 g / ml c. .5 g / ml d. 8 ml / g 27. How many Hydrogen atoms are in the following ...
... 24. Plasmas include all of the following except: a. ionized gases b. lava c. lightning d. stars 26. If the mass of a steel bolt is 4.0 grams and its volume is 2 milliliters, what is the bolt’s density? a. 2 ml / g b. 2 g / ml c. .5 g / ml d. 8 ml / g 27. How many Hydrogen atoms are in the following ...
Atomic nucleus
The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The atomic nucleus was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.The diameter of the nucleus is in the range of 6985175000000000000♠1.75 fm (6985175000000000000♠1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 6986150000000000000♠15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.