Sunday, September 14, 2003 غامر الحكيم 057403435 حمود العويد
... In 1887, Not able to detect the earth’s motion through the ether. Albert Einstein German-American - 1879-1955. No ether In 1905, Theory of relativity Max Plank German- 1858-1947 atom emits light in discrete energy - photons In 1900, he was able to derive the correct blackbody spectrum by assuming th ...
... In 1887, Not able to detect the earth’s motion through the ether. Albert Einstein German-American - 1879-1955. No ether In 1905, Theory of relativity Max Plank German- 1858-1947 atom emits light in discrete energy - photons In 1900, he was able to derive the correct blackbody spectrum by assuming th ...
Light
... The History of the Photoelectric effect In late 19th century it was discovered that electrons were emitted from a zinc plate when it was exposed to ultraviolet (UV) radiation. As visible light and UV are electromagnetic (EM) waves of similar nature, the result suggests that EM radiation carries ener ...
... The History of the Photoelectric effect In late 19th century it was discovered that electrons were emitted from a zinc plate when it was exposed to ultraviolet (UV) radiation. As visible light and UV are electromagnetic (EM) waves of similar nature, the result suggests that EM radiation carries ener ...
Ch. 2 Chemistry
... • Protons, which are positively charged • Electrons, which are negatively charged ...
... • Protons, which are positively charged • Electrons, which are negatively charged ...
Name - mrshayden
... The amount of energy E, measured in ergs, is based on the amount of ground motion recorded by a seismograph at a known distance from the epicenter of the quake. The logarithmic function for the Richter scale assigns very large numbers for the amount of energy, E, to numbers that range from 1 to 9. A ...
... The amount of energy E, measured in ergs, is based on the amount of ground motion recorded by a seismograph at a known distance from the epicenter of the quake. The logarithmic function for the Richter scale assigns very large numbers for the amount of energy, E, to numbers that range from 1 to 9. A ...
Differences between Electron and Ions Linacs
... Higher frequencies are economically convenient (shorter, less RF power, higher gradients possible) but the limitation comes from mechanical precision required in construction (tight tolerances are expensive!) and beam dynamics for ion linacs. Electron linacs tend to use higher frequencies than ion l ...
... Higher frequencies are economically convenient (shorter, less RF power, higher gradients possible) but the limitation comes from mechanical precision required in construction (tight tolerances are expensive!) and beam dynamics for ion linacs. Electron linacs tend to use higher frequencies than ion l ...
Chapter 7 Ionic and Metallic Bonding
... inner (core) electrons Put one dot for each valence electron (8 maximum) They don’t pair up until they have to (Hund’s rule) ...
... inner (core) electrons Put one dot for each valence electron (8 maximum) They don’t pair up until they have to (Hund’s rule) ...
Franca pITTALUGA - Università Iuav di Venezia
... façade, which creates a constantly changing speckled light internally, mutating according to the degree of granulation of the gabions. Jean Nouvel’s sophisticated application of a superimposed photo-sensitive screen at the Institute of the Arab world in Paris derives from the world of optics; the co ...
... façade, which creates a constantly changing speckled light internally, mutating according to the degree of granulation of the gabions. Jean Nouvel’s sophisticated application of a superimposed photo-sensitive screen at the Institute of the Arab world in Paris derives from the world of optics; the co ...
Reflection from a potential step (PPT - 8.5MB)
... Although the amplitude of the wave is smaller after the barrier, no energy is lost in the tunneling process ...
... Although the amplitude of the wave is smaller after the barrier, no energy is lost in the tunneling process ...
What are Kinetic and Potential Energy?
... to the ball. As it flies over the net, the ball has kinetic energy. • Kinetic energy is the energy of motion. • All moving objects have kinetic energy. • Like all forms of energy, kinetic energy can be used to do work. For example, kinetic energy allows a hammer to do work on a nail. ...
... to the ball. As it flies over the net, the ball has kinetic energy. • Kinetic energy is the energy of motion. • All moving objects have kinetic energy. • Like all forms of energy, kinetic energy can be used to do work. For example, kinetic energy allows a hammer to do work on a nail. ...
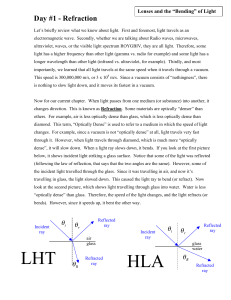
Refraction - fwiatrowskimbhs
... A ray of light is approaching a set of three mirrors as shown in the diagram. The light ray is approaching the first mirror at an angle of 45-degrees with the mirror surface. Trace the path of the light ray as it bounces off the mirror. Continue tracing the ray until it finally exits from the mirror ...
... A ray of light is approaching a set of three mirrors as shown in the diagram. The light ray is approaching the first mirror at an angle of 45-degrees with the mirror surface. Trace the path of the light ray as it bounces off the mirror. Continue tracing the ray until it finally exits from the mirror ...
File
... 112. The empirical formula of a compound is CH2. The molecular formula of this compound could be A) CH 4 B) C2H2 C) C2H 4 D) C2H6 113. Two basic properties of the gas phase are A) a definite shape and a definite volume B) a definite shape but no definite volume C) no definite shape but a definite vo ...
... 112. The empirical formula of a compound is CH2. The molecular formula of this compound could be A) CH 4 B) C2H2 C) C2H 4 D) C2H6 113. Two basic properties of the gas phase are A) a definite shape and a definite volume B) a definite shape but no definite volume C) no definite shape but a definite vo ...