1 Elementary Particle Mass-Radius Relationships S. Reucroft* and
... The photon might also be a fundamental particle. All other elementary particles are composite objects made of combinations of electrons and neutrinos bound by gravity. ...
... The photon might also be a fundamental particle. All other elementary particles are composite objects made of combinations of electrons and neutrinos bound by gravity. ...
12 - RosedaleGrade10Science
... 2. The figure below represents a beam of light going from one medium to another. One medium is air, in which light has a speed of 3.0 x 108 m/s. The other medium is ice, in which light has a speed of 2.29 x 108 m/s. Identify which medium below is ice and air. Explain. ...
... 2. The figure below represents a beam of light going from one medium to another. One medium is air, in which light has a speed of 3.0 x 108 m/s. The other medium is ice, in which light has a speed of 2.29 x 108 m/s. Identify which medium below is ice and air. Explain. ...
IOSR Journal of Electrical and Electronics Engineering (IOSR-JEEE)
... Photovoltaic, the conversion of sunlight to electricity, are a promising technology that may allow the generation of electrical power on a very large scale. It works on the basic principle of photovoltaic effect where when a photon of energy hv which is equal or greater than the energy gap, is absor ...
... Photovoltaic, the conversion of sunlight to electricity, are a promising technology that may allow the generation of electrical power on a very large scale. It works on the basic principle of photovoltaic effect where when a photon of energy hv which is equal or greater than the energy gap, is absor ...
Chem BIG REVIEW - Jones-wiki
... FUSION – 2 or more small nuclei combine to one larger nucleus FISSION – 1 large nucleus splits to form two or more smaller nuclei A half-life is the time required for one-half of a radioisotope’s nuclei to decay into its products. The half-life of any particular radioisotope is constant and therefor ...
... FUSION – 2 or more small nuclei combine to one larger nucleus FISSION – 1 large nucleus splits to form two or more smaller nuclei A half-life is the time required for one-half of a radioisotope’s nuclei to decay into its products. The half-life of any particular radioisotope is constant and therefor ...
Document
... Atoms and molecules can exist only in certain energy states. In each energy state, the atom or molecule has a definite energy. When an atom or molecule changes its energy state, it must emit or absorb just enough energy to bring it to the new energy state (the quantum condition). Atoms or molecules ...
... Atoms and molecules can exist only in certain energy states. In each energy state, the atom or molecule has a definite energy. When an atom or molecule changes its energy state, it must emit or absorb just enough energy to bring it to the new energy state (the quantum condition). Atoms or molecules ...
Objective 5 - Physics
... Sound acts like other waves • Echoes are reflected sound waves • Sonar uses echoes to judge distance to ...
... Sound acts like other waves • Echoes are reflected sound waves • Sonar uses echoes to judge distance to ...
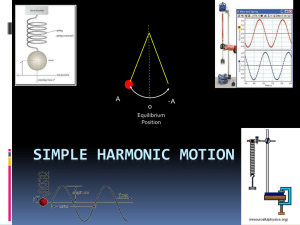
Simple Harmonic motion
... Now move your hand so that is matches (or comes close to) the natural frequency) of the slinky. It will start to oscillate with greater and greater amplitude. ...
... Now move your hand so that is matches (or comes close to) the natural frequency) of the slinky. It will start to oscillate with greater and greater amplitude. ...
Multi-electron correlation spectroscopy of atoms and molecules
... known to us today as the photoelectric effect was made by Heinrich Rudolf Hertz in 1887, who explored that the incidence of ultra-violet (UV) light on a spark gap facilitated the passage of the electrical spark. Later on, Aleksandr Stoletov discovered the direct proportionality between the intensity ...
... known to us today as the photoelectric effect was made by Heinrich Rudolf Hertz in 1887, who explored that the incidence of ultra-violet (UV) light on a spark gap facilitated the passage of the electrical spark. Later on, Aleksandr Stoletov discovered the direct proportionality between the intensity ...