17.1 Physics 6B Electric Potential
... When the potential energy of the system decreases, positive work is done by the electric force. When potential energy increases, negative work is done by the electric force (or alternatively, Prepared by Vince Zaccone positive work is done on the system by outside forces). For Campus Learning Assist ...
... When the potential energy of the system decreases, positive work is done by the electric force. When potential energy increases, negative work is done by the electric force (or alternatively, Prepared by Vince Zaccone positive work is done on the system by outside forces). For Campus Learning Assist ...
Document
... Shower shape analysis is used for /0 discrimination. Reduction in near angle peak towards photon Bin. Effect is more prominent for larger Ettrigger . Away-side yield is reduced. ...
... Shower shape analysis is used for /0 discrimination. Reduction in near angle peak towards photon Bin. Effect is more prominent for larger Ettrigger . Away-side yield is reduced. ...
... Processing polyacetylene and many other polymers such as polypyrrole and polythiophene was for a time ruled out because of their failure to melt or to dissolve in any solvent. Ingenious methods developed over the years have, however, made processing possible. In 1980, James W. Feast and co-workers a ...
Atom The smallest part of an element that can exist on its own
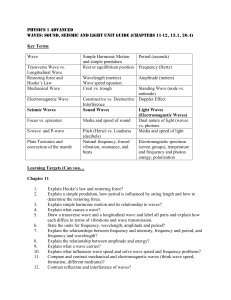
... Dibasic acid One which has 2 replaceable H atoms per molecule Isotopes Atoms having the same atomic number but different mass numbers - As the number of protons increases, the number of neutrons increases relatively faster, so small atoms have proton and neutron numbers which are comparable whereas ...
... Dibasic acid One which has 2 replaceable H atoms per molecule Isotopes Atoms having the same atomic number but different mass numbers - As the number of protons increases, the number of neutrons increases relatively faster, so small atoms have proton and neutron numbers which are comparable whereas ...
chapter 5.
... electron, the orbital electron is ejected from its shell and the resulting orbital vacancy is filled by an electron from a higher level shell. The energy difference between the two shells may be emitted from the atom either in the form of a characteristic photon (characteristic x-ray) or is transfer ...
... electron, the orbital electron is ejected from its shell and the resulting orbital vacancy is filled by an electron from a higher level shell. The energy difference between the two shells may be emitted from the atom either in the form of a characteristic photon (characteristic x-ray) or is transfer ...
L3_interactions_matter_riegler09 - Indico
... If a particle propagates in a material with a velocity larger than the speed of light in this material, Cherenkov radiation is emitted at a characteristic angle that depends on the particle velocity and the refractive index of the material. Transition Radiation: If a charged particle is crossing the ...
... If a particle propagates in a material with a velocity larger than the speed of light in this material, Cherenkov radiation is emitted at a characteristic angle that depends on the particle velocity and the refractive index of the material. Transition Radiation: If a charged particle is crossing the ...
Electrostatics
... a. The center of the negative cloud normally coincides with the center of normally coincides with the center of the positive nucleus in an atom. b. When an external negative charge is g g brought nearby to the right, the electron cloud is distorted so that the centers of negative and positive ...
... a. The center of the negative cloud normally coincides with the center of normally coincides with the center of the positive nucleus in an atom. b. When an external negative charge is g g brought nearby to the right, the electron cloud is distorted so that the centers of negative and positive ...
Slide 1
... If the spring is pulled down and released it will oscillate. The frequency of this oscillation is called natural frequency. Definition: Natural frequency is the frequency an object will vibrate with after an external disturbance. All objects have a natural frequency or set of frequencies - at which ...
... If the spring is pulled down and released it will oscillate. The frequency of this oscillation is called natural frequency. Definition: Natural frequency is the frequency an object will vibrate with after an external disturbance. All objects have a natural frequency or set of frequencies - at which ...
Chemical Bond Activation Observed with an X
... crystal to oxygen at about room temperature according to the recipe from Lizzit et al.15 The sample was placed at a grazing incidence angle of approximately 2° into the soft X-ray beam and the collinear 400 nm pump laser beam both at 120 Hz and focused to a spot size of about 50 μm by 50 μm. It was ...
... crystal to oxygen at about room temperature according to the recipe from Lizzit et al.15 The sample was placed at a grazing incidence angle of approximately 2° into the soft X-ray beam and the collinear 400 nm pump laser beam both at 120 Hz and focused to a spot size of about 50 μm by 50 μm. It was ...
Accelerator Experiments and Theoretical Models for the Electron Screening Effect in
... 2 Accelerator experiments 2.1 Set-up and data acquisition and analysis The experiments have been carried out at an accelerator optimized for low energy beams. Fig. 1 illustrates the principal set-up and the data acquisition system. The accelerator consists of a radio frequency ion source, an accele ...
... 2 Accelerator experiments 2.1 Set-up and data acquisition and analysis The experiments have been carried out at an accelerator optimized for low energy beams. Fig. 1 illustrates the principal set-up and the data acquisition system. The accelerator consists of a radio frequency ion source, an accele ...
4471 Session 4: Numerical Simulations
... • Caution: the total electronic energy is not the sum of the Hartree-Fock eigenvalues (this would double-count the electron-electron interaction terms) • The difficulty: the exchange term is quite difficult to evaluate since it is non-local • Still have no account at all of correlation between elect ...
... • Caution: the total electronic energy is not the sum of the Hartree-Fock eigenvalues (this would double-count the electron-electron interaction terms) • The difficulty: the exchange term is quite difficult to evaluate since it is non-local • Still have no account at all of correlation between elect ...