3.10 Neutralization
... ZnS(s) + 2HCl(aq) → ZnCl2(aq) + H2S(g) ZnS(s) + 2H+ + 2Cl- → Zn2+ + 2Cl- + H2S(g) ⇒ZnS(s) + 2H+ → Zn2+ + H2S(g) – H+ is present in the form of H3O+ ...
... ZnS(s) + 2HCl(aq) → ZnCl2(aq) + H2S(g) ZnS(s) + 2H+ + 2Cl- → Zn2+ + 2Cl- + H2S(g) ⇒ZnS(s) + 2H+ → Zn2+ + H2S(g) – H+ is present in the form of H3O+ ...
Phase-I metabolism
... Oxidative Phase-I involving cytochrome P-450 enzymes: • N-oxidation: – Mostly for primary and secondary amines as well as aromatic amines: – This gives N-oxide that will be rapidly converted to ...
... Oxidative Phase-I involving cytochrome P-450 enzymes: • N-oxidation: – Mostly for primary and secondary amines as well as aromatic amines: – This gives N-oxide that will be rapidly converted to ...
ENZYME Test REVIEW Answers
... 9. What is the optimal pH for trypsin? 8.5 11. Is this pH acid or basic 12. Neither enzyme works at a pHs of__5_ 13. An incomplete graph is shown below. What two internal body conditions could appropriately be used to replace letter Z on the axis? pH or temperature ...
... 9. What is the optimal pH for trypsin? 8.5 11. Is this pH acid or basic 12. Neither enzyme works at a pHs of__5_ 13. An incomplete graph is shown below. What two internal body conditions could appropriately be used to replace letter Z on the axis? pH or temperature ...
An Introduction to Metabolism
... • The cell’s proteins harness the energy released by hydrolysis of ATP to perform the 3 types of cellular work discussed (mechanical, transport, and chemical) – With the help of specific enzymes, cells can use the energy released by hydrolysis of ATP to drive endergonic reactions • If ΔG of endergon ...
... • The cell’s proteins harness the energy released by hydrolysis of ATP to perform the 3 types of cellular work discussed (mechanical, transport, and chemical) – With the help of specific enzymes, cells can use the energy released by hydrolysis of ATP to drive endergonic reactions • If ΔG of endergon ...
Chapter 7 7 The Behavior of Proteins: Enzymes Mechanisms and
... h reaction i off structurally related substrates to give structurally related l d products d • Stereospecificity: catalyzes a reaction in which one stereoisomer is reacted or formed in p preference to all others that might be reacted or formed ...
... h reaction i off structurally related substrates to give structurally related l d products d • Stereospecificity: catalyzes a reaction in which one stereoisomer is reacted or formed in p preference to all others that might be reacted or formed ...
on the enzyme
... place where the target molecule can attach. This place is called the active site. The target molecule/chemical is the substrate. ...
... place where the target molecule can attach. This place is called the active site. The target molecule/chemical is the substrate. ...
Ch 6 Enzymes and Metabolism - Liberty Union High School District
... reduce the amount of energy to start a reaction Pheeew… that takes a lot less energy! ...
... reduce the amount of energy to start a reaction Pheeew… that takes a lot less energy! ...
08_Lecture_Presentation_PC
... The Activation Energy Barrier • Every chemical reaction between molecules involves bond breaking and bond forming • The initial energy needed to start a chemical reaction is called the free energy of activation, or activation energy (EA) • Activation energy is often supplied in the form of thermal ...
... The Activation Energy Barrier • Every chemical reaction between molecules involves bond breaking and bond forming • The initial energy needed to start a chemical reaction is called the free energy of activation, or activation energy (EA) • Activation energy is often supplied in the form of thermal ...
Evolution & organisation of metabolic Pathways
... • DEB model is closed under symbiogenesis: it is possible to model symbiogenesis of two initially independently living populations that follow the DEB rules by incremental changes of parameter values such that a single population emerges that again follows the DEB rules • essential property for mode ...
... • DEB model is closed under symbiogenesis: it is possible to model symbiogenesis of two initially independently living populations that follow the DEB rules by incremental changes of parameter values such that a single population emerges that again follows the DEB rules • essential property for mode ...
Lec 15: Nitrogen in biochemistry
... • NH3 ammonia is the most important nitrogen compound that almost all life could use and is vital for crop production. However, biological N2 fixation is limited in rate as N=N is extremely stable. • In 1909 – Fritz Haber invented the direct chemical synthesis of NH3 from N2 + H2 in lab. immediately ...
... • NH3 ammonia is the most important nitrogen compound that almost all life could use and is vital for crop production. However, biological N2 fixation is limited in rate as N=N is extremely stable. • In 1909 – Fritz Haber invented the direct chemical synthesis of NH3 from N2 + H2 in lab. immediately ...
Document
... Electron Transport Chain • Food (glucose) is oxidized and the hydrogen: • Are transported by coenzymes NADH and FADH2 • Enter a chain of proteins bound to metal atoms (cofactors) • Combine with molecular oxygen to form water • Release energy ...
... Electron Transport Chain • Food (glucose) is oxidized and the hydrogen: • Are transported by coenzymes NADH and FADH2 • Enter a chain of proteins bound to metal atoms (cofactors) • Combine with molecular oxygen to form water • Release energy ...
Enzyme Activity
... Inhibitors are chemicals that reduce the rate of enzymic reactions. The are usually specific and they work at low concentrations. They block the enzyme but they do not usually destroy it. ...
... Inhibitors are chemicals that reduce the rate of enzymic reactions. The are usually specific and they work at low concentrations. They block the enzyme but they do not usually destroy it. ...
BOROUGH OF MANHATTAN COMMUNITY COLLEGE City
... one’s own creation. Using the idea or work of another is permissible only when the original author is identified. Paraphrasing and summarizing, as well as direct quotations, require citations to the original source. Plagiarism may be intentional or unintentional. Lack of dishonest intent does not ne ...
... one’s own creation. Using the idea or work of another is permissible only when the original author is identified. Paraphrasing and summarizing, as well as direct quotations, require citations to the original source. Plagiarism may be intentional or unintentional. Lack of dishonest intent does not ne ...
C485 Exam I
... 1. (6pts) Which of the following statement about cyclic photophosphorylation are correct? (Circle the correct ones) A) It doesn’t involve NADPH formation B) It uses electrons supplied by photosystem II C) It is activated when NADP+ is limiting D) It does not generate O2 E) It leads to ATP production ...
... 1. (6pts) Which of the following statement about cyclic photophosphorylation are correct? (Circle the correct ones) A) It doesn’t involve NADPH formation B) It uses electrons supplied by photosystem II C) It is activated when NADP+ is limiting D) It does not generate O2 E) It leads to ATP production ...
Name 1 BIO 451 14 December, 1998 FINAL EXAM
... Patsteur observed that the rate and extent of glucose consumption by yeast under anaerobic and aerobic conditions were very different. Which condition (anaerobic or aerobic) would be associated with the greatest rate of glucose consumption? (2 point) The greatest glucose consumption would be under a ...
... Patsteur observed that the rate and extent of glucose consumption by yeast under anaerobic and aerobic conditions were very different. Which condition (anaerobic or aerobic) would be associated with the greatest rate of glucose consumption? (2 point) The greatest glucose consumption would be under a ...
Section 5: Enzymes, Equilibrium, Energy and the
... (dihydrofolic acid, DHF) is increased. The efficacy of trimethoprim will be decreased. With more of the normal substrate present, it is more likely to bind to the target enzyme, decreasing the effect of trimethoprim. 2. Compare and contrast the interaction of β-lactams with transpeptidase and that o ...
... (dihydrofolic acid, DHF) is increased. The efficacy of trimethoprim will be decreased. With more of the normal substrate present, it is more likely to bind to the target enzyme, decreasing the effect of trimethoprim. 2. Compare and contrast the interaction of β-lactams with transpeptidase and that o ...
View Full PDF
... [10], enzymes that belong to a protein superfamily that contains human 1 l,-hydroxysteroid dehydrogenase, 17,/-hydroxysteroid dehydrogenase, 1 5-hydroxyprostaglandin dehydrogenase and Drosophila melanogaster alcohol dehydrogenase, as well as bacterial enzymes that are important in synthesis of antib ...
... [10], enzymes that belong to a protein superfamily that contains human 1 l,-hydroxysteroid dehydrogenase, 17,/-hydroxysteroid dehydrogenase, 1 5-hydroxyprostaglandin dehydrogenase and Drosophila melanogaster alcohol dehydrogenase, as well as bacterial enzymes that are important in synthesis of antib ...
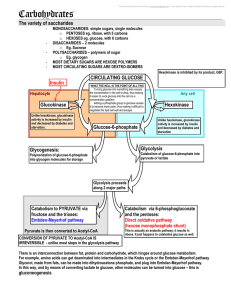
5 carbohydrates and the Krebs Cycle
... you go down the Embden-Meyerhof pathway or the hexose monophosphate shunt pathway. o Embden-Meyerhof pathway produces 4 mol of ATP per mol of glucose, and uses up 1 mol. The end product is phosphoglyceraldehyde o This is an ANAEROBIC process o Thus, there is a net gain of 3 mol of ATP o However, w ...
... you go down the Embden-Meyerhof pathway or the hexose monophosphate shunt pathway. o Embden-Meyerhof pathway produces 4 mol of ATP per mol of glucose, and uses up 1 mol. The end product is phosphoglyceraldehyde o This is an ANAEROBIC process o Thus, there is a net gain of 3 mol of ATP o However, w ...
Bio_Ch2_ Enzymes_2009
... • Some chemical reactions that make life possible are too slow or have activation energies that are too high to make them practical for living tissue • Enzyme—catalytic protein that speeds up the chemical reactions by lowering the activation energy (end with –ase) ...
... • Some chemical reactions that make life possible are too slow or have activation energies that are too high to make them practical for living tissue • Enzyme—catalytic protein that speeds up the chemical reactions by lowering the activation energy (end with –ase) ...
September 27 AP Biology - John D. O`Bryant School of Math & Science
... Do Now (Quiz) Five dialysis bags, constructed from a semi-permeable membrane that is impermeable to sucrose, were filled with various concentrations of sucrose and then placed in separate beakers containing an initial concentration of 0.6 M sucrose solution. At 10-minute intervals, the bags were ...
... Do Now (Quiz) Five dialysis bags, constructed from a semi-permeable membrane that is impermeable to sucrose, were filled with various concentrations of sucrose and then placed in separate beakers containing an initial concentration of 0.6 M sucrose solution. At 10-minute intervals, the bags were ...
Oxidative phosphorylation
Oxidative phosphorylation (or OXPHOS in short) is the metabolic pathway in which the mitochondria in cells use their structure, enzymes, and energy released by the oxidation of nutrients to reform ATP. Although the many forms of life on earth use a range of different nutrients, ATP is the molecule that supplies energy to metabolism. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is probably so pervasive because it is a highly efficient way of releasing energy, compared to alternative fermentation processes such as anaerobic glycolysis.During oxidative phosphorylation, electrons are transferred from electron donors to electron acceptors such as oxygen, in redox reactions. These redox reactions release energy, which is used to form ATP. In eukaryotes, these redox reactions are carried out by a series of protein complexes within the inner membrane of the cell's mitochondria, whereas, in prokaryotes, these proteins are located in the cells' intermembrane space. These linked sets of proteins are called electron transport chains. In eukaryotes, five main protein complexes are involved, whereas in prokaryotes many different enzymes are present, using a variety of electron donors and acceptors.The energy released by electrons flowing through this electron transport chain is used to transport protons across the inner mitochondrial membrane, in a process called electron transport. This generates potential energy in the form of a pH gradient and an electrical potential across this membrane. This store of energy is tapped by allowing protons to flow back across the membrane and down this gradient, through a large enzyme called ATP synthase; this process is known as chemiosmosis. This enzyme uses this energy to generate ATP from adenosine diphosphate (ADP), in a phosphorylation reaction. This reaction is driven by the proton flow, which forces the rotation of a part of the enzyme; the ATP synthase is a rotary mechanical motor.Although oxidative phosphorylation is a vital part of metabolism, it produces reactive oxygen species such as superoxide and hydrogen peroxide, which lead to propagation of free radicals, damaging cells and contributing to disease and, possibly, aging (senescence). The enzymes carrying out this metabolic pathway are also the target of many drugs and poisons that inhibit their activities.