Lecture of Enzymes.
... In the Figure B, this is illustrated schematically using a bond-breaking enzyme as an example. Type A (top): Highly specific enzymes catalyze the cleavage of only one type of bond, and only when the structure of the substrate is the correct one. Type B (middle): Other enzymes have narrow reaction sp ...
... In the Figure B, this is illustrated schematically using a bond-breaking enzyme as an example. Type A (top): Highly specific enzymes catalyze the cleavage of only one type of bond, and only when the structure of the substrate is the correct one. Type B (middle): Other enzymes have narrow reaction sp ...
oxidation numbers
... 1 Work out the formula of the species before and after the change; 2 If different numbers of the relevant species are on both sides, balance them 3 Work out the oxidation number of the element before and after the change 4 Add electrons to one side of the equation so the oxidation numbers balance 5 ...
... 1 Work out the formula of the species before and after the change; 2 If different numbers of the relevant species are on both sides, balance them 3 Work out the oxidation number of the element before and after the change 4 Add electrons to one side of the equation so the oxidation numbers balance 5 ...
Metabolic Pathways and Energy Production
... Match the following terms with the descriptions: 1) Catabolic reactions 2) Coenzymes 3) Glycolysis 4) Lactate A. 4 Produced during anaerobic conditions. B. 3 Reaction series that converts glucose to pyruvate. C. 1 Metabolic reactions that break down large molecules to smaller molecules + energy. D. ...
... Match the following terms with the descriptions: 1) Catabolic reactions 2) Coenzymes 3) Glycolysis 4) Lactate A. 4 Produced during anaerobic conditions. B. 3 Reaction series that converts glucose to pyruvate. C. 1 Metabolic reactions that break down large molecules to smaller molecules + energy. D. ...
Anaerobic Energy Systems
... (oxygen is required for full breakdown). 2 molecules of ATP are produced (18 times less than aerobic!). Lactic acid is produced as a by-product. This system can therefore only be sustained for between 10 seconds and 3 mins. Few chemical reactions involved so energy can be produced quickly. summary o ...
... (oxygen is required for full breakdown). 2 molecules of ATP are produced (18 times less than aerobic!). Lactic acid is produced as a by-product. This system can therefore only be sustained for between 10 seconds and 3 mins. Few chemical reactions involved so energy can be produced quickly. summary o ...
Anaerobic Energy Systems
... (oxygen is required for full breakdown). 2 molecules of ATP are produced (18 times less than aerobic!). Lactic acid is produced as a by-product. This system can therefore only be sustained for between 10 seconds and 3 mins. Few chemical reactions involved so energy can be produced quickly. summary o ...
... (oxygen is required for full breakdown). 2 molecules of ATP are produced (18 times less than aerobic!). Lactic acid is produced as a by-product. This system can therefore only be sustained for between 10 seconds and 3 mins. Few chemical reactions involved so energy can be produced quickly. summary o ...
oxidation–reduction reaction
... compound is the number of electrons lost or gained by the atom when it forms ions. • Oxidation numbers are tools that scientists use in written chemical equations to help them keep track of the movement of electrons in a redox reaction. ...
... compound is the number of electrons lost or gained by the atom when it forms ions. • Oxidation numbers are tools that scientists use in written chemical equations to help them keep track of the movement of electrons in a redox reaction. ...
Hemoglobin and Cytochrome c
... subunits resemble both themselves and myoglobin. The subunits are symmetrically arranged. Note the extensive contacts at between alpha and beta subunits, while there is little alpha-alpha and beta-beta contact (as they face each other across a 20 Å channel that runs through the center of the protein ...
... subunits resemble both themselves and myoglobin. The subunits are symmetrically arranged. Note the extensive contacts at between alpha and beta subunits, while there is little alpha-alpha and beta-beta contact (as they face each other across a 20 Å channel that runs through the center of the protein ...
Document
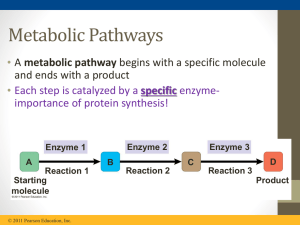
... oxygen) • Anabolic pathways consume energy to build complex molecules from simpler ones (synthesis of protein from amino acids) ...
... oxygen) • Anabolic pathways consume energy to build complex molecules from simpler ones (synthesis of protein from amino acids) ...
Enzymes: Introduction notes
... back reaction? What is the rate enhancement brought about by the catalyst for that reaction? Draw the free energy diagram of a hypothetical reaction and show how a catalyst may increase the rate of the reaction, pointing out on the diagram ∆G for the overall reaction, ∆G‡uncat, and ∆G‡cat. Indicate ...
... back reaction? What is the rate enhancement brought about by the catalyst for that reaction? Draw the free energy diagram of a hypothetical reaction and show how a catalyst may increase the rate of the reaction, pointing out on the diagram ∆G for the overall reaction, ∆G‡uncat, and ∆G‡cat. Indicate ...
Biochemistry - Text of NPTEL IIT Video Lectures
... compound. The electrons from the tricarboxylic acid cycle are made available to an electron transport chain in the form of 3 NADH and 1 FADH2. So these are also formed in the reactions in the Krebs cycle and this NADH and FADH2 is utilized in oxidative phosphorylation for the production of ATP where ...
... compound. The electrons from the tricarboxylic acid cycle are made available to an electron transport chain in the form of 3 NADH and 1 FADH2. So these are also formed in the reactions in the Krebs cycle and this NADH and FADH2 is utilized in oxidative phosphorylation for the production of ATP where ...
Respiration
... Glucose converted to pyruvate This is called glycolysis Glycolysis occurs in cytoplasm Energy is released to produce two ATP No oxygen present Pyruvate broken down to carbon dioxide and ethanol • Incomplete breakdown of glucose/irriversible ...
... Glucose converted to pyruvate This is called glycolysis Glycolysis occurs in cytoplasm Energy is released to produce two ATP No oxygen present Pyruvate broken down to carbon dioxide and ethanol • Incomplete breakdown of glucose/irriversible ...
lect21
... bind to the enzyme separately, the reaction is randomorder ternary type -in most cases the rate of the first reaction is 10 – 100 times the rate of the second reactions, but in some enzymes the rates are nearly equal ...
... bind to the enzyme separately, the reaction is randomorder ternary type -in most cases the rate of the first reaction is 10 – 100 times the rate of the second reactions, but in some enzymes the rates are nearly equal ...
The Frog Cell Cycle
... Once a cell initiates mitosis, why does it never slip back into S or G2? ...
... Once a cell initiates mitosis, why does it never slip back into S or G2? ...
Lecture 38 - Amino Acid Metabolism 1
... Nitrogen fixation takes place in bacteria and is the primary process by which atmospheric N2 gas is converted to ammonia (NH4+) and nitrogen oxides (NO2- and NO3-) in the biosphere. Nitrogen assimilation incorporates this ammonia into amino acids, primarily glutamate and glutamine. 2. What are the n ...
... Nitrogen fixation takes place in bacteria and is the primary process by which atmospheric N2 gas is converted to ammonia (NH4+) and nitrogen oxides (NO2- and NO3-) in the biosphere. Nitrogen assimilation incorporates this ammonia into amino acids, primarily glutamate and glutamine. 2. What are the n ...
Document
... Entry of other carbohydrates into glycolysis Fructose Liver Cells They have another enzyme, fructokinase. • It has a stronger affinity for fructose. • It catalyzes phosphoryl group transfer from ATP to produce fructose-1phosphate. An aldolase-type cleavage and additional phosphorylation must also o ...
... Entry of other carbohydrates into glycolysis Fructose Liver Cells They have another enzyme, fructokinase. • It has a stronger affinity for fructose. • It catalyzes phosphoryl group transfer from ATP to produce fructose-1phosphate. An aldolase-type cleavage and additional phosphorylation must also o ...
Final a
... 4. (10 pts) List the environmental conditions/small molecules that activate rubisco and/or enzymes of the Calvin cycle. ...
... 4. (10 pts) List the environmental conditions/small molecules that activate rubisco and/or enzymes of the Calvin cycle. ...
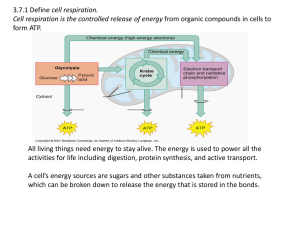
3.7:Cell Respiration Aerobic cell respiration: glucose
... cell respiration produces energy; controlled release of energy; by breakdown of organic molecules/glucose; energy from them is used to make ATP; aerobic respiration is in mitochondria; requires oxygen; pyruvate is produced by glycolysis / glucose broken down; pyruvate is broken down in the mitochond ...
... cell respiration produces energy; controlled release of energy; by breakdown of organic molecules/glucose; energy from them is used to make ATP; aerobic respiration is in mitochondria; requires oxygen; pyruvate is produced by glycolysis / glucose broken down; pyruvate is broken down in the mitochond ...
Solution
... The R groups of amino acid residues within the active site of an enzyme bind the substrate by forming hydrogen bonds, salt bridges, and hydrophobic interactions and catalyze the reaction. ...
... The R groups of amino acid residues within the active site of an enzyme bind the substrate by forming hydrogen bonds, salt bridges, and hydrophobic interactions and catalyze the reaction. ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 06. Native gel is used to isolate enzymes. 07. Starch is a homopoloysaccharide. 08. The dipeptide contains one peptide bond. 09. Saturated fatty acids contain double bond. 10. IUB refers to International Union of Biochemistry. III. Complete the following ...
... 06. Native gel is used to isolate enzymes. 07. Starch is a homopoloysaccharide. 08. The dipeptide contains one peptide bond. 09. Saturated fatty acids contain double bond. 10. IUB refers to International Union of Biochemistry. III. Complete the following ...
Fatty Acid Oxidation and Ketone Bodies OXIDATION OF FATTY
... Fatty acids are both oxidized to acetyl-CoA and synthesized from acetyl-CoA. Although the staring material of one process is identical to the product of the other, fatty acid oxidation is not the simple reverse of fatty acid biosynthesis. It is an entirely different process taking place in separate ...
... Fatty acids are both oxidized to acetyl-CoA and synthesized from acetyl-CoA. Although the staring material of one process is identical to the product of the other, fatty acid oxidation is not the simple reverse of fatty acid biosynthesis. It is an entirely different process taking place in separate ...
Bio9A Quiz 1 Study Guide
... reflect doubling of reactions because of 2C3 molecules. 2. These ATPs are made by substrate level phosphorylation v. Pyruvate moves to mitochondria. b. Acetyl-CoA formation (Fig 7.9) i. Yields 2 NADH (1 per pyruvate) ii. Occurs in matrix of mitochondria. iii. Pyruvate + CoA CO2 + Acetyl-CoA c. Cit ...
... reflect doubling of reactions because of 2C3 molecules. 2. These ATPs are made by substrate level phosphorylation v. Pyruvate moves to mitochondria. b. Acetyl-CoA formation (Fig 7.9) i. Yields 2 NADH (1 per pyruvate) ii. Occurs in matrix of mitochondria. iii. Pyruvate + CoA CO2 + Acetyl-CoA c. Cit ...
Oxidative phosphorylation
Oxidative phosphorylation (or OXPHOS in short) is the metabolic pathway in which the mitochondria in cells use their structure, enzymes, and energy released by the oxidation of nutrients to reform ATP. Although the many forms of life on earth use a range of different nutrients, ATP is the molecule that supplies energy to metabolism. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is probably so pervasive because it is a highly efficient way of releasing energy, compared to alternative fermentation processes such as anaerobic glycolysis.During oxidative phosphorylation, electrons are transferred from electron donors to electron acceptors such as oxygen, in redox reactions. These redox reactions release energy, which is used to form ATP. In eukaryotes, these redox reactions are carried out by a series of protein complexes within the inner membrane of the cell's mitochondria, whereas, in prokaryotes, these proteins are located in the cells' intermembrane space. These linked sets of proteins are called electron transport chains. In eukaryotes, five main protein complexes are involved, whereas in prokaryotes many different enzymes are present, using a variety of electron donors and acceptors.The energy released by electrons flowing through this electron transport chain is used to transport protons across the inner mitochondrial membrane, in a process called electron transport. This generates potential energy in the form of a pH gradient and an electrical potential across this membrane. This store of energy is tapped by allowing protons to flow back across the membrane and down this gradient, through a large enzyme called ATP synthase; this process is known as chemiosmosis. This enzyme uses this energy to generate ATP from adenosine diphosphate (ADP), in a phosphorylation reaction. This reaction is driven by the proton flow, which forces the rotation of a part of the enzyme; the ATP synthase is a rotary mechanical motor.Although oxidative phosphorylation is a vital part of metabolism, it produces reactive oxygen species such as superoxide and hydrogen peroxide, which lead to propagation of free radicals, damaging cells and contributing to disease and, possibly, aging (senescence). The enzymes carrying out this metabolic pathway are also the target of many drugs and poisons that inhibit their activities.