Alpha oxidation
... • They are disorders metabolism of fatty acids, branched chain and aromatic amino acids and citric acid cycle. • The incidence of medium chain acyl coA dehydrogenase deficiency is about 1 in 2500 live birth, and is the second most common inborn error of metabolism. • They are all characterised by th ...
... • They are disorders metabolism of fatty acids, branched chain and aromatic amino acids and citric acid cycle. • The incidence of medium chain acyl coA dehydrogenase deficiency is about 1 in 2500 live birth, and is the second most common inborn error of metabolism. • They are all characterised by th ...
Ch. 7 Study Guide
... □ I can explain how glucose is oxidized during glycolysis and the Krebs Cycle to produce reducing power in NADH and FADH ...
... □ I can explain how glucose is oxidized during glycolysis and the Krebs Cycle to produce reducing power in NADH and FADH ...
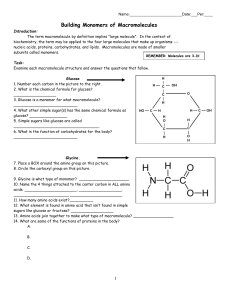
Building Monomers of Macromolecules
... Fatty acid 21. Place a BOX around the hydrocarbon chain in these pictures. 22. Circle the carboxyl group in both pictures. 23. Fatty acids are made of long chains of _______________ atoms with attached ______________ atoms. 24. What 3 elements make up fatty acids? _________________ 25. How are satur ...
... Fatty acid 21. Place a BOX around the hydrocarbon chain in these pictures. 22. Circle the carboxyl group in both pictures. 23. Fatty acids are made of long chains of _______________ atoms with attached ______________ atoms. 24. What 3 elements make up fatty acids? _________________ 25. How are satur ...
Using Computational Chemistry to Determine the Fate of Organic
... Perflourooctanoic acid (PFOA) and other perfluorinated alkyl acids (PFAAs) have been found distributed across soil, air and groundwater around the world. These compounds have been widely used as polymerization precursors of fluoropolymers such as PTFE (polytetrafluoroethylene, or Teflon©). PFAAs and ...
... Perflourooctanoic acid (PFOA) and other perfluorinated alkyl acids (PFAAs) have been found distributed across soil, air and groundwater around the world. These compounds have been widely used as polymerization precursors of fluoropolymers such as PTFE (polytetrafluoroethylene, or Teflon©). PFAAs and ...
Enzymes & Energy
... transferred to ADP, phosphorylating it to ATP. 2 more ATPs are made. Two pyruvic acid molecules are formed from the single original glucose. ...
... transferred to ADP, phosphorylating it to ATP. 2 more ATPs are made. Two pyruvic acid molecules are formed from the single original glucose. ...
2- All essential amino acids are glucogenic. False
... An increase in the availability of gluconeogenic amino acids from the catabolism of body protein is associated with increased ammonia and results in increased urea production. ...
... An increase in the availability of gluconeogenic amino acids from the catabolism of body protein is associated with increased ammonia and results in increased urea production. ...
Paper - IndiaStudyChannel.com
... (A) stimulates the activity of acetyl CoA carboxylase (B) is important for fatty acid oxidation (C) inhibits the formation of triacylglycerol (D) none of these 29. In the major pathway by which liver produces ketone bodies, the immediate precursor of acetoacetate is : (A) acetoacetyl CoA (B) -hydro ...
... (A) stimulates the activity of acetyl CoA carboxylase (B) is important for fatty acid oxidation (C) inhibits the formation of triacylglycerol (D) none of these 29. In the major pathway by which liver produces ketone bodies, the immediate precursor of acetoacetate is : (A) acetoacetyl CoA (B) -hydro ...
Citric acid cycle • What are the functions of Citric Acid Cycle?
... A triacylglycerol is a glycerol esterified with three fatty acids. The fatty acids can be saturated, mono-unsaturated or poly-unsaturated. Usually they have an even number of carbon atoms (12-20). ...
... A triacylglycerol is a glycerol esterified with three fatty acids. The fatty acids can be saturated, mono-unsaturated or poly-unsaturated. Usually they have an even number of carbon atoms (12-20). ...
IE EA
... f) SF6 Neither; the coordination number of six is rarely exceeded so that this molecule does not act as a Lewis acid and the high electronegativity of fluorine does not allow for it to act as a base. g) PCl5 Acidic; this compound reacts with a wide variety of Lewis bases to form adducts. h) (CH3)3N ...
... f) SF6 Neither; the coordination number of six is rarely exceeded so that this molecule does not act as a Lewis acid and the high electronegativity of fluorine does not allow for it to act as a base. g) PCl5 Acidic; this compound reacts with a wide variety of Lewis bases to form adducts. h) (CH3)3N ...
PYRUVATE DEHYDROGENASE COMPLEX
... amino acid degradation is not significant normally, but neurons consume amino acids instead of synthesizing proteins in this case Other organs can degrade fatty acids efficiently, therefore they are not damaged. d) Acetylcholine neurotransmitter sythesis requires acetyl-CoA, produced by PDHC. ...
... amino acid degradation is not significant normally, but neurons consume amino acids instead of synthesizing proteins in this case Other organs can degrade fatty acids efficiently, therefore they are not damaged. d) Acetylcholine neurotransmitter sythesis requires acetyl-CoA, produced by PDHC. ...
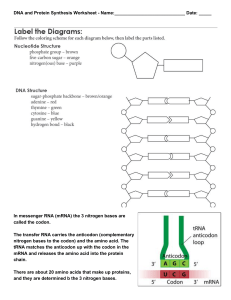
File
... chain. There are about 20 amino acids that make up proteins, and they are determined b the 3 nitrogen bases. ...
... chain. There are about 20 amino acids that make up proteins, and they are determined b the 3 nitrogen bases. ...
The Preparation of Fragrant Esters
... CH3-COOH + HO-CH2CH3 CH3-COO-CH2CH3 + H2O The ester product of this reaction (CH3-COO-CH2CH3) is named ethyl acetate, indicating the acid and alcohol from which it is prepared. Esterification is an equilibrium reaction, which means that the reaction does not go to completion on its own. Frequently ...
... CH3-COOH + HO-CH2CH3 CH3-COO-CH2CH3 + H2O The ester product of this reaction (CH3-COO-CH2CH3) is named ethyl acetate, indicating the acid and alcohol from which it is prepared. Esterification is an equilibrium reaction, which means that the reaction does not go to completion on its own. Frequently ...
Amino acid An organic compound containing both an
... Compounds which contain the elements carbon, hydrogen and oxygen; there is always twice as much hydrogen as there is oxygen. Made up of sub units called simple sugars; carbohydrates are one of the major classes of nutrients; one function in the body is as an energy source. ...
... Compounds which contain the elements carbon, hydrogen and oxygen; there is always twice as much hydrogen as there is oxygen. Made up of sub units called simple sugars; carbohydrates are one of the major classes of nutrients; one function in the body is as an energy source. ...
Exam 3 - Chemistry Courses: About
... E. Hydrolysis of a high energy bond in succinyl CoA leads to formation of this compound: (Draw the structure below.) ...
... E. Hydrolysis of a high energy bond in succinyl CoA leads to formation of this compound: (Draw the structure below.) ...
1. Which of the following is not a feature of scientific hypotheses? A
... A) is toxic to all forms of life. B) can be used in place of oxygen. C) blocks much ultraviolet radiation. D) provides energy to some basic forms of life. E) disinfects. ...
... A) is toxic to all forms of life. B) can be used in place of oxygen. C) blocks much ultraviolet radiation. D) provides energy to some basic forms of life. E) disinfects. ...
Butyrate formation from glucose by the rumen protozoon Dasytricha
... hydro-lyase, 3-hydroxyacyl-CoA reductase, phosphate butyryltransferase and butyrate kinase. Subcellular fractionation by differential and density-gradient centrifugation on sucrose gradients indicated that all those enzymes except pyruvate: ferredoxin oxidoreductase were non-sedimentable at 6 x 106g ...
... hydro-lyase, 3-hydroxyacyl-CoA reductase, phosphate butyryltransferase and butyrate kinase. Subcellular fractionation by differential and density-gradient centrifugation on sucrose gradients indicated that all those enzymes except pyruvate: ferredoxin oxidoreductase were non-sedimentable at 6 x 106g ...
WEB
... Understand differences and similarities between starvation and diabetes Describe the reactions of fatty acid synthesis ...
... Understand differences and similarities between starvation and diabetes Describe the reactions of fatty acid synthesis ...
Cell Respiration Notes (Honors)
... These new molecules are broken down to form ATP and CO2. One ATP per cycle is produced, two cycles occur per glucose molecule – therefore 2 ATP’s are produced by Krebs Cycle. *Also generates high energy electrons carried by NADH and FADH2. ...
... These new molecules are broken down to form ATP and CO2. One ATP per cycle is produced, two cycles occur per glucose molecule – therefore 2 ATP’s are produced by Krebs Cycle. *Also generates high energy electrons carried by NADH and FADH2. ...
Chapter 29 Biosynthetic Pathways 308 29.1 Your text states in
... (b) The number of glucose residues may be as high as 1,000,000. 29.13 Uridine triphosphate (UTP) is a nucleoside triphosphate similar to ATP. The constituents are: a nitrogen base, uracil; a sugar, ribose; and three phosphates. 29.14 The carbon atoms used in fatty acid synthesis have their origin in ...
... (b) The number of glucose residues may be as high as 1,000,000. 29.13 Uridine triphosphate (UTP) is a nucleoside triphosphate similar to ATP. The constituents are: a nitrogen base, uracil; a sugar, ribose; and three phosphates. 29.14 The carbon atoms used in fatty acid synthesis have their origin in ...
Year 12 AS Biology Module 1: Biological Molecules Name: PAPER
... Calculate the Rf value of spot X. Show your working. ...
... Calculate the Rf value of spot X. Show your working. ...
Chapter 21 Biosynthetic Pathways
... • These sources are most commonly pyruvate, citric acid cycle intermediates, and glucogenic amino acids. • Gluconeogenesis is not the exact reversal of glycolysis; that is, pyruvate to glucose does not occur by reversing the steps of glucose to pyruvate. • There are three irreversible steps in glyco ...
... • These sources are most commonly pyruvate, citric acid cycle intermediates, and glucogenic amino acids. • Gluconeogenesis is not the exact reversal of glycolysis; that is, pyruvate to glucose does not occur by reversing the steps of glucose to pyruvate. • There are three irreversible steps in glyco ...
Chapter 9: Cellular Respiration
... 9-1 Chemical Pathways Chemical Energy and Food • Cellular respiration happens ________ and in many _______. • If all the energy was release in one step . . . Most would be lost as _______ and _______! Cellular respiration breaks down _________ molecules and banks their energy in ________. Photosyn ...
... 9-1 Chemical Pathways Chemical Energy and Food • Cellular respiration happens ________ and in many _______. • If all the energy was release in one step . . . Most would be lost as _______ and _______! Cellular respiration breaks down _________ molecules and banks their energy in ________. Photosyn ...
print
... • Nitriles also have dipole-dipole interactions, because they have a polar C≡N group. • Because they contain one or two N–H bonds, 1° and 2° amides are capable of intermolecular hydrogen bonding and will have substantially higher melting and boiling points. ...
... • Nitriles also have dipole-dipole interactions, because they have a polar C≡N group. • Because they contain one or two N–H bonds, 1° and 2° amides are capable of intermolecular hydrogen bonding and will have substantially higher melting and boiling points. ...
NO OXYGEN!
... when oxygen is not available. For example, in muscle tissues during rapid and vigorous exercise, muscle cells may be depleted of oxygen. They then switch from respiration to fermentation. ...
... when oxygen is not available. For example, in muscle tissues during rapid and vigorous exercise, muscle cells may be depleted of oxygen. They then switch from respiration to fermentation. ...
Butyric acid
Butyric acid (from Greek βούτῡρον, meaning ""butter""), also known under the systematic name butanoic acid, abbreviated BTA, is a carboxylic acid with the structural formula CH3CH2CH2-COOH. Salts and esters of butyric acid are known as butyrates or butanoates. Butyric acid is found in milk, especially goat, sheep and buffalo milk, butter, parmesan cheese, and as a product of anaerobic fermentation (including in the colon and as body odor). It has an unpleasant smell and acrid taste, with a sweetish aftertaste (similar to ether). It can be detected by mammals with good scent detection abilities (such as dogs) at 10 parts per billion, whereas humans can detect it in concentrations above 10 parts per million.Butyric acid is present in, and is the main distinctive smell of, human vomit.Butyric acid was first observed (in impure form) in 1814 by the French chemist Michel Eugène Chevreul. By 1818, he had purified it sufficiently to characterize it. The name of butyric acid comes from the Latin word for butter, butyrum (or buturum), the substance in which butyric acid was first found.