Available
... production of urea is more than recovered by the release of energy formed during the synthesis of the urea cycle intermediates. Ammonia released during the glutamate dehydrogenase reaction is coupled to the formation of NADH. In addition, when fumarate is converted back to aspartate, the malate dehy ...
... production of urea is more than recovered by the release of energy formed during the synthesis of the urea cycle intermediates. Ammonia released during the glutamate dehydrogenase reaction is coupled to the formation of NADH. In addition, when fumarate is converted back to aspartate, the malate dehy ...
Prentice Hall Biology
... Oxygen is the final electron ________ and combines with hydrogen to form water Go to Section: ...
... Oxygen is the final electron ________ and combines with hydrogen to form water Go to Section: ...
Energy metabolism
... cholesterol. However, plasma epinephrine and serum free fatty acid concentrations were increased (P < 0[middle dot]05). In the third experiment, BM(7.5 and 15 g/kg) and 1[middle dot]5 % BM lowered triacylglycerol concentration in red gastrocnemius and tibialis anterior (P < 0[middle dot]05) muscle, ...
... cholesterol. However, plasma epinephrine and serum free fatty acid concentrations were increased (P < 0[middle dot]05). In the third experiment, BM(7.5 and 15 g/kg) and 1[middle dot]5 % BM lowered triacylglycerol concentration in red gastrocnemius and tibialis anterior (P < 0[middle dot]05) muscle, ...
Metabolism of fat File
... completely to acetyl-CoA (C2 units). In the case of palmitic acid the reactions are repeated 7 times and 8 molecules of acetyl CoA are formed. Since acetyl-CoA can be oxidized to CO2 and water via the citric acid cycle, the complete oxidation of fatty acids is achieved ...
... completely to acetyl-CoA (C2 units). In the case of palmitic acid the reactions are repeated 7 times and 8 molecules of acetyl CoA are formed. Since acetyl-CoA can be oxidized to CO2 and water via the citric acid cycle, the complete oxidation of fatty acids is achieved ...
LEARNING OBJECTIVES Vitamin B12, Folic Acid and B6 • To
... When B12 is deficient these fatty acids accumulate in cell membrane of C.N.S Causing Neurologic symptoms. ...
... When B12 is deficient these fatty acids accumulate in cell membrane of C.N.S Causing Neurologic symptoms. ...
Fermentation PowerPoint File
... Fermentation is a process by which energy can be released from food molecules in the absence of oxygen. Fermentation occurs in the cytoplasm of cells. ...
... Fermentation is a process by which energy can be released from food molecules in the absence of oxygen. Fermentation occurs in the cytoplasm of cells. ...
Al - Iraqia university/ college of medicine
... plant origin (e.g., corn oil & soybean oil), are liquid at room temperature. Fat uses: Long-term energy storage. Insulates against heat loss. Forms protective cushion around organs. Steroids: Smaller lipid molecules function as chemical messengers. Emulsifiers: cause fats to mix with water. Th ...
... plant origin (e.g., corn oil & soybean oil), are liquid at room temperature. Fat uses: Long-term energy storage. Insulates against heat loss. Forms protective cushion around organs. Steroids: Smaller lipid molecules function as chemical messengers. Emulsifiers: cause fats to mix with water. Th ...
LAB-AIDS^ #505-12 Molecules ot Lite Kit Student
... Carbohydrates, fats, proteins and nucleic acid's are the four major groups of organic molecules found in living organisms. This Lab-Aids kit deals with the important class of organic molecules known as proteins. They are the main structural and growth components of cells in tissues such as skin, hai ...
... Carbohydrates, fats, proteins and nucleic acid's are the four major groups of organic molecules found in living organisms. This Lab-Aids kit deals with the important class of organic molecules known as proteins. They are the main structural and growth components of cells in tissues such as skin, hai ...
American-Journal-of-Oil-and-Chemical-Technologies
... According to research conducted in our research group on the pyridinedicarboxylatefamily due to their various applications, we decided to oxygenate nitrogen of pyridine ring of pyridine-2,5-dicarboxylic acid as N-oxide to investigate synthesis, coordination modes, and structure types of these compou ...
... According to research conducted in our research group on the pyridinedicarboxylatefamily due to their various applications, we decided to oxygenate nitrogen of pyridine ring of pyridine-2,5-dicarboxylic acid as N-oxide to investigate synthesis, coordination modes, and structure types of these compou ...
Revision PPT on enzymes File
... Why is shape important? The shape of an enzyme is very important because it has a direct effect on how it catalyzes a reaction. Why do enzymes have different shapes? An enzyme’s shape is determined by the sequence of amino acids in its structure, and the bonds which form between the atoms of those ...
... Why is shape important? The shape of an enzyme is very important because it has a direct effect on how it catalyzes a reaction. Why do enzymes have different shapes? An enzyme’s shape is determined by the sequence of amino acids in its structure, and the bonds which form between the atoms of those ...
amino acids
... Why is shape important? The shape of an enzyme is very important because it has a direct effect on how it catalyzes a reaction. Why do enzymes have different shapes? An enzyme’s shape is determined by the sequence of amino acids in its structure, and the bonds which form between the atoms of those ...
... Why is shape important? The shape of an enzyme is very important because it has a direct effect on how it catalyzes a reaction. Why do enzymes have different shapes? An enzyme’s shape is determined by the sequence of amino acids in its structure, and the bonds which form between the atoms of those ...
Slide 1
... (AKA the Citric Acid Cycle) • Occurs in the mitochondria within the mitochondrial matrix and requires oxygen. • Pyruvic acid (made from Glycolysis) is broken down into citric acid. • Citric Acid is broken down and releases CO2 during each step of the cycle. • ATP is also created • Also releases more ...
... (AKA the Citric Acid Cycle) • Occurs in the mitochondria within the mitochondrial matrix and requires oxygen. • Pyruvic acid (made from Glycolysis) is broken down into citric acid. • Citric Acid is broken down and releases CO2 during each step of the cycle. • ATP is also created • Also releases more ...
Anaerobic Respiration
... lactic acid and energy is released (which is used to form ATP). Glucose → pyruvate → Lactic acid + energy ...
... lactic acid and energy is released (which is used to form ATP). Glucose → pyruvate → Lactic acid + energy ...
ABBREVIATIONS IN ASPET JOURNALS
... Abstract. In general, nonstandard abbreviations are allowed in the abstract if used twice or more. Write out the definition the first time. Write out the full chemical name for numbered compounds (e.g., PD98059); Place the designation in parentheses after the written-out version even if it is used o ...
... Abstract. In general, nonstandard abbreviations are allowed in the abstract if used twice or more. Write out the definition the first time. Write out the full chemical name for numbered compounds (e.g., PD98059); Place the designation in parentheses after the written-out version even if it is used o ...
CHAPTER 3: CELL STRUCTURE AND FUNCTION
... Energy-Investment Steps Two ATP are used to activate glucose as glycolysis begins. Energy-Harvesting Steps Glycolysis breaks down glucose to two molecules of pyruvate, making ATP by substratelevel ATP synthesis. There is a net gain of 2 ATP from glycolysis. 7.4 Inside the Mitochondria Preparatory Re ...
... Energy-Investment Steps Two ATP are used to activate glucose as glycolysis begins. Energy-Harvesting Steps Glycolysis breaks down glucose to two molecules of pyruvate, making ATP by substratelevel ATP synthesis. There is a net gain of 2 ATP from glycolysis. 7.4 Inside the Mitochondria Preparatory Re ...
Fatty Acid Metabolism - Weber State University
... The presence of both glucose and ketone bodies in the urine are a strong indication of diabetes. Lack of sufficient insulin results in high blood sugar levels that spill glucose into the urine. Since cells are not stimulated to absorb glucose so they must resort to fatty acid oxidation. Low levels o ...
... The presence of both glucose and ketone bodies in the urine are a strong indication of diabetes. Lack of sufficient insulin results in high blood sugar levels that spill glucose into the urine. Since cells are not stimulated to absorb glucose so they must resort to fatty acid oxidation. Low levels o ...
4.6 Fermentation
... 1. Cells contain only enough ATP for a few seconds of intense activity 2. Then cells rely on lactic acid fermentation (can supply for about 90 seconds) 3. Lactic acid build-up causes burning in muscles. Only way to get rid of lactic acid is chemical pathway that requires oxygen (why you breathe heav ...
... 1. Cells contain only enough ATP for a few seconds of intense activity 2. Then cells rely on lactic acid fermentation (can supply for about 90 seconds) 3. Lactic acid build-up causes burning in muscles. Only way to get rid of lactic acid is chemical pathway that requires oxygen (why you breathe heav ...
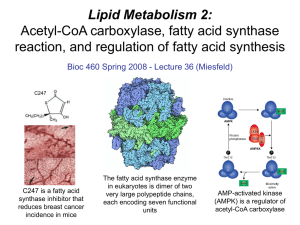
Lecture 36 - Lipid Metabolism 2
... using a biotin-mediated reaction mechanism that carboxylates acetyl-CoA to form the C3 compound malonyl-CoA.. Fatty acid synthase - this large multi-functional enzyme is responsible for catalyzing a series of reactions that sequentially adds C2 units to a growing fatty acid chain covalently attached ...
... using a biotin-mediated reaction mechanism that carboxylates acetyl-CoA to form the C3 compound malonyl-CoA.. Fatty acid synthase - this large multi-functional enzyme is responsible for catalyzing a series of reactions that sequentially adds C2 units to a growing fatty acid chain covalently attached ...
Midterm Exam Advanced Biochemistry II (Answer) 1. At equilibrium
... of glycolysis by two reactions, promoted by phosphoglycerate kinase and pyruvate kinase. Suppose skeletal muscle were devoid of lactate dehydrogenase. Could it carry out strenuous physical activity; that is, could it generate ATP at a high rate by glycolysis? Explain. Answer The key point here is th ...
... of glycolysis by two reactions, promoted by phosphoglycerate kinase and pyruvate kinase. Suppose skeletal muscle were devoid of lactate dehydrogenase. Could it carry out strenuous physical activity; that is, could it generate ATP at a high rate by glycolysis? Explain. Answer The key point here is th ...
TSTH Cleanse Foods to Consume
... Nettle Leaf – has a great number of amino acids, panthotenic acid, folic acid, chlorophyll. It also contains vitamins C, B2 and K, beta-carotene, Calcium, Magnesium, and Iron. Because of these compounds, the plant has anti-anemic, anti-diabetic, haemostatic and diuretic properties Milk Thistle – ant ...
... Nettle Leaf – has a great number of amino acids, panthotenic acid, folic acid, chlorophyll. It also contains vitamins C, B2 and K, beta-carotene, Calcium, Magnesium, and Iron. Because of these compounds, the plant has anti-anemic, anti-diabetic, haemostatic and diuretic properties Milk Thistle – ant ...
SATL-POC - Systematic Approach to Teaching
... units. The absorption of O-H stretching appears as a broad band near 3000 cm-1. The νC=O stretching absorption in aliphatic acids occurs at 1725-1700 cm-1. • Some of the acids viz., acetic acid, benzoic acid, exist as dimmers due to hydrogen bonding. Formation of bridge lowers the force constants an ...
... units. The absorption of O-H stretching appears as a broad band near 3000 cm-1. The νC=O stretching absorption in aliphatic acids occurs at 1725-1700 cm-1. • Some of the acids viz., acetic acid, benzoic acid, exist as dimmers due to hydrogen bonding. Formation of bridge lowers the force constants an ...
cell energy test review
... MATCHING QUESTIONS From the list below, select the term that best fits each description. Each term may be used more than once, but there is only one correct answer for each description. Questions 1—5 refer to the following: a. electron transport b. glycolysis ...
... MATCHING QUESTIONS From the list below, select the term that best fits each description. Each term may be used more than once, but there is only one correct answer for each description. Questions 1—5 refer to the following: a. electron transport b. glycolysis ...
Butyric acid
Butyric acid (from Greek βούτῡρον, meaning ""butter""), also known under the systematic name butanoic acid, abbreviated BTA, is a carboxylic acid with the structural formula CH3CH2CH2-COOH. Salts and esters of butyric acid are known as butyrates or butanoates. Butyric acid is found in milk, especially goat, sheep and buffalo milk, butter, parmesan cheese, and as a product of anaerobic fermentation (including in the colon and as body odor). It has an unpleasant smell and acrid taste, with a sweetish aftertaste (similar to ether). It can be detected by mammals with good scent detection abilities (such as dogs) at 10 parts per billion, whereas humans can detect it in concentrations above 10 parts per million.Butyric acid is present in, and is the main distinctive smell of, human vomit.Butyric acid was first observed (in impure form) in 1814 by the French chemist Michel Eugène Chevreul. By 1818, he had purified it sufficiently to characterize it. The name of butyric acid comes from the Latin word for butter, butyrum (or buturum), the substance in which butyric acid was first found.