Chapter 5
... • Zeff is lower than actual nuclear charge. • Zeff increases toward nucleus ns > np > nd > nf • This explains certain periodic changes observed. ...
... • Zeff is lower than actual nuclear charge. • Zeff increases toward nucleus ns > np > nd > nf • This explains certain periodic changes observed. ...
Chapter 7 - Quantum Numbers, Orbitals, and Electron
... The H atom orbitals may be used to approximate the orbitals for multi-electron atoms. But since these atoms have more than one electron, electrons in the outer orbitals are shielded somewhat from the nucleus: they do not feel the full nuclear charge. Orbitals with a lower l value penetrate closer to ...
... The H atom orbitals may be used to approximate the orbitals for multi-electron atoms. But since these atoms have more than one electron, electrons in the outer orbitals are shielded somewhat from the nucleus: they do not feel the full nuclear charge. Orbitals with a lower l value penetrate closer to ...
Name: Period
... 3. What are the shapes of an s and p orbitals? 4. What is a principal energy level, sublevel and atomic orbital? 5. What is the maximum number in each s, p, d and f orbitals? 6. What types of atomic orbitals are in the 1st, 2nd and 3rd principal energy levels? 7. If the spin of one electron is clock ...
... 3. What are the shapes of an s and p orbitals? 4. What is a principal energy level, sublevel and atomic orbital? 5. What is the maximum number in each s, p, d and f orbitals? 6. What types of atomic orbitals are in the 1st, 2nd and 3rd principal energy levels? 7. If the spin of one electron is clock ...
Atoms and Elements Notes
... 1. Physical Change- A change in form that does not result in a new substance. Ex. Sugar dissolves in water or any state change. 2. Chemical Change- A change/reaction that creates a new substance. An indication this is happening is when you see a color change, heat, light, smoke/gas and a new byprodu ...
... 1. Physical Change- A change in form that does not result in a new substance. Ex. Sugar dissolves in water or any state change. 2. Chemical Change- A change/reaction that creates a new substance. An indication this is happening is when you see a color change, heat, light, smoke/gas and a new byprodu ...
Chapter 3 notes
... Electron Arrangement in Atoms Hund’s rule – When electrons occupy orbitals of equal energy, one electron enters each orbital until all orbitals contain one electron with parallel spins Once all orbitals of equal energy have one electron with parallel spin, the next electron to enter the orbital h ...
... Electron Arrangement in Atoms Hund’s rule – When electrons occupy orbitals of equal energy, one electron enters each orbital until all orbitals contain one electron with parallel spins Once all orbitals of equal energy have one electron with parallel spin, the next electron to enter the orbital h ...
Transition metal configurations and limitations of the orbital
... k~ectronfunctions. This approximation &Ids hydrogenic orbitals whose energies may be obtained exactly. Clearly the orbital energies ob&ed in this way will fail to correspond to observed data on ionization energies in which inter-electron repulsions are a contributing factor. The Hartree-Fock method ...
... k~ectronfunctions. This approximation &Ids hydrogenic orbitals whose energies may be obtained exactly. Clearly the orbital energies ob&ed in this way will fail to correspond to observed data on ionization energies in which inter-electron repulsions are a contributing factor. The Hartree-Fock method ...
The quantum theory was used to show how the wavelike behavior of
... Remember that an orbital can hold 0, 1, or 2 electrons only, and if there are two electrons in the orbital, they must have opposite (paired) spins (Pauli principle ) 3. within a subshell (depicted as a group of boxes), spread the electrons out and line up their spins as much as possible (Hund's rule ...
... Remember that an orbital can hold 0, 1, or 2 electrons only, and if there are two electrons in the orbital, they must have opposite (paired) spins (Pauli principle ) 3. within a subshell (depicted as a group of boxes), spread the electrons out and line up their spins as much as possible (Hund's rule ...
Science 9
... A) are composed of metal ions bonded to other metal ions. B) are formed when metal react with non-metals. C) are substances with low melting points. D) are usually insoluble in water. 3. Molecular compounds… A) are combinations of metals and non-metals. B) form when electrons are shared. C) are good ...
... A) are composed of metal ions bonded to other metal ions. B) are formed when metal react with non-metals. C) are substances with low melting points. D) are usually insoluble in water. 3. Molecular compounds… A) are combinations of metals and non-metals. B) form when electrons are shared. C) are good ...
Honors Chemistry Semester 1 Exam Review
... 3. Isotopes are atoms of the same element, which have the same number of (protons / neutrons) but a different number (protons / neutrons). 4. How do isotopes C-12 and C-14 differ from each other? ________________________________________________ How are they similar? _________________________________ ...
... 3. Isotopes are atoms of the same element, which have the same number of (protons / neutrons) but a different number (protons / neutrons). 4. How do isotopes C-12 and C-14 differ from each other? ________________________________________________ How are they similar? _________________________________ ...
Definitions are in Book
... 4) How do neon lights work? Once the light is plugged in and turned on, the electricity causes the electrons in neon to become ‘excited’, which means the electrons move a higher energy level orbital. Once they’re there, they want to get back down to ground state (the orbital they were originally in) ...
... 4) How do neon lights work? Once the light is plugged in and turned on, the electricity causes the electrons in neon to become ‘excited’, which means the electrons move a higher energy level orbital. Once they’re there, they want to get back down to ground state (the orbital they were originally in) ...
HW-1-Ch1-Atomic-structure-W16
... 35. Penetration & Shielding of an Electron in multi-electron atom and how does it affect the filling order as given by “Building Up” principle? ...
... 35. Penetration & Shielding of an Electron in multi-electron atom and how does it affect the filling order as given by “Building Up” principle? ...
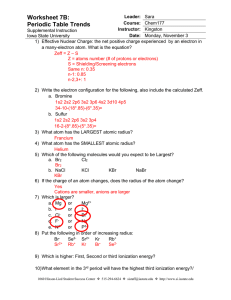
worksheet 7b answers - Iowa State University
... Iowa State University 1) Effective Nuclear Charge: the net positive charge experienced by an electron in a many-electron atom. What is the equation? Zeff = Z – S Z = atoms number (# of protons or electrons) S = Shielding/Screening electrons Same n: 0.35 n-1: 0.85 n-2,3+: 1 ...
... Iowa State University 1) Effective Nuclear Charge: the net positive charge experienced by an electron in a many-electron atom. What is the equation? Zeff = Z – S Z = atoms number (# of protons or electrons) S = Shielding/Screening electrons Same n: 0.35 n-1: 0.85 n-2,3+: 1 ...
Quantum Notes
... Ground vs. Excited Ground state is when the electrons are in the lowest energy levels. Electrons that have absorbed energy jump to higher levels and become excited. ...
... Ground vs. Excited Ground state is when the electrons are in the lowest energy levels. Electrons that have absorbed energy jump to higher levels and become excited. ...
Periodic Table Jeopardy
... Atomic Theory with evidence. He had four key postulates that he wanted everyone to know. ...
... Atomic Theory with evidence. He had four key postulates that he wanted everyone to know. ...
Metal Questions
... 10. Which electrons are lost by an atom of iron when it forms the Fe3ion? A. One s orbital electron and two d orbital electrons B. Two s orbital electrons and one d orbital electron C. Three s orbital electrons D. Three d orbital electrons (2000) Give the electronic configuration of the d-block el ...
... 10. Which electrons are lost by an atom of iron when it forms the Fe3ion? A. One s orbital electron and two d orbital electrons B. Two s orbital electrons and one d orbital electron C. Three s orbital electrons D. Three d orbital electrons (2000) Give the electronic configuration of the d-block el ...
Atoms, electrons, nuclei J.J. Thomson discovered the electron (1897
... energy Ek to overcome the work eUanode: Ek ≥ eUanode electron collide with many Mercury atoms, if Ugrid < U*, these collisions will always be elastic: no energy loss and anode current (I) will increase; if Ugrid = U*, collisions might become inelastic: electrons may transfer their energy to a Mercur ...
... energy Ek to overcome the work eUanode: Ek ≥ eUanode electron collide with many Mercury atoms, if Ugrid < U*, these collisions will always be elastic: no energy loss and anode current (I) will increase; if Ugrid = U*, collisions might become inelastic: electrons may transfer their energy to a Mercur ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.