(n=1).
... • Bohr’s Model gives accurate values for electron energy levels... • But Quantum Mechanics is needed to describe electrons in atom. • Electrons jump between states by emitting or absorbing photons of the appropriate energy. • Each state has specific energy and is labeled by 4 quantum numbers (next t ...
... • Bohr’s Model gives accurate values for electron energy levels... • But Quantum Mechanics is needed to describe electrons in atom. • Electrons jump between states by emitting or absorbing photons of the appropriate energy. • Each state has specific energy and is labeled by 4 quantum numbers (next t ...
Corso di Fisica Moderna
... With the use of spectroscopy in the late 19th century, it was found that the radiaAon from hydrogen, as well as other atoms, was emiNed at specific quanAzed frequencies. It was the effort to exp ...
... With the use of spectroscopy in the late 19th century, it was found that the radiaAon from hydrogen, as well as other atoms, was emiNed at specific quanAzed frequencies. It was the effort to exp ...
Unit Description - Honors Chemistry
... Explain the quantum concept of EMR energy (5.1) Explain how an element can be identified by its spectral lines (5.1) Explain the origin of the atomic emission spectrum of an element, using Bohr’s hydrogen spectrum (5.1) Describe the quantum mechanical model of the atom (5.2) Describe Heise ...
... Explain the quantum concept of EMR energy (5.1) Explain how an element can be identified by its spectral lines (5.1) Explain the origin of the atomic emission spectrum of an element, using Bohr’s hydrogen spectrum (5.1) Describe the quantum mechanical model of the atom (5.2) Describe Heise ...
The true nature of the atom?
... Yea, but is this quantum stuff really relevant? Does all this quantum stuff really matter outside of the classroom and lab? Yep, we’re about to look at the development of the quantum mechanical behavior of the atom, a theoretical framework that expresses the behavior of matter at the atomic scale. ...
... Yea, but is this quantum stuff really relevant? Does all this quantum stuff really matter outside of the classroom and lab? Yep, we’re about to look at the development of the quantum mechanical behavior of the atom, a theoretical framework that expresses the behavior of matter at the atomic scale. ...
unit 7 hw packet File
... F. Describe electron motion based on the Schrödinger equation and the Heisenberg uncertainty principle. G. Describe the photoelectric effect and how it relates to atomic structure and the dual nature of light. H. Identify and compare electron orbitals (s, p, d, f). I. Write electron configurations f ...
... F. Describe electron motion based on the Schrödinger equation and the Heisenberg uncertainty principle. G. Describe the photoelectric effect and how it relates to atomic structure and the dual nature of light. H. Identify and compare electron orbitals (s, p, d, f). I. Write electron configurations f ...
ki̇mya
... • Electrons move in each stationary energy state in a circular orbital. These circular orbitals are called energy levels or shells. The possible states for the electron are numbered, n=1, 2, 3 and so on. • When an electron is in a stationary state, the atom does not emit light. However when an elect ...
... • Electrons move in each stationary energy state in a circular orbital. These circular orbitals are called energy levels or shells. The possible states for the electron are numbered, n=1, 2, 3 and so on. • When an electron is in a stationary state, the atom does not emit light. However when an elect ...
Ch. 5 PPT Part 2
... that it is fundamentally impossible to know precisely both the velocity and position of an electron at the same time. • The only quantity that can be known is the probability for an electron to occupy a certain region around the nucleus. ...
... that it is fundamentally impossible to know precisely both the velocity and position of an electron at the same time. • The only quantity that can be known is the probability for an electron to occupy a certain region around the nucleus. ...
ap chemistry review – multiple choice
... Questions 15-18 refer to the following (a) Heisenbery uncertainty principle (b) Pauli exclusion principle (c) Hund’s rule (principle of maximum multiplicity) (d) Shielding effect (e) Wave nature of matter 15. Can be used to predict that a gaseous carbon atom in it ground state is paramagnetic 16. E ...
... Questions 15-18 refer to the following (a) Heisenbery uncertainty principle (b) Pauli exclusion principle (c) Hund’s rule (principle of maximum multiplicity) (d) Shielding effect (e) Wave nature of matter 15. Can be used to predict that a gaseous carbon atom in it ground state is paramagnetic 16. E ...
Advanced Chemistry Midterm
... 36. What are parts of the electromagnetic spectrum in order from lowest frequency/lowest energy to highest frequency/highest energy? ...
... 36. What are parts of the electromagnetic spectrum in order from lowest frequency/lowest energy to highest frequency/highest energy? ...
Chapter 6.8 - Periodic Trends
... Look up the word trend in the dictionary. (See online dictionary.) A trend is _____. a. a rule or behavior which is always followed b. a general tendency to behave in a certain manner c. the tendency to be modern or current The periodic law was introduced in Chapter 2. The law states that _____. a. ...
... Look up the word trend in the dictionary. (See online dictionary.) A trend is _____. a. a rule or behavior which is always followed b. a general tendency to behave in a certain manner c. the tendency to be modern or current The periodic law was introduced in Chapter 2. The law states that _____. a. ...
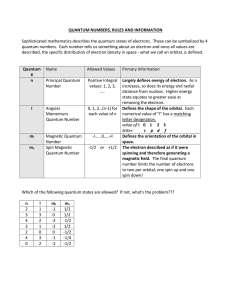
Quantum Number Table
... Largely defines energy of electron. As n increases, so does its energy and radial distance from nucleus. Higher energy state equates to greater ease at removing the electron. Defines the shape of the orbital. Each numerical value of "l" has a matching letter designation. value of l: 0 1 2 3 letter: ...
... Largely defines energy of electron. As n increases, so does its energy and radial distance from nucleus. Higher energy state equates to greater ease at removing the electron. Defines the shape of the orbital. Each numerical value of "l" has a matching letter designation. value of l: 0 1 2 3 letter: ...
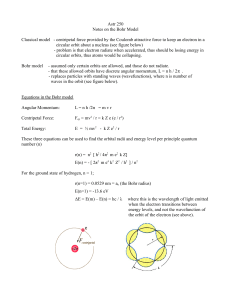
Astr 250 Notes on the Bohr Model Classical model
... circular orbit about a nucleus (see figure below) - problem is that electron radiate when accelerated, thus should be losing energy in circular orbits, thus atoms would be collapsing. Bohr model ...
... circular orbit about a nucleus (see figure below) - problem is that electron radiate when accelerated, thus should be losing energy in circular orbits, thus atoms would be collapsing. Bohr model ...
Student - Davison Chemistry Website
... 3. Each level has a certain amount of energy associated with it and the electrons can only jump levels if they gain or lose energy 4. Lowest energy levels closest to nucleus a. In the ____________________________ for an atom, electrons are at their lowest, most stable energy levels. ...
... 3. Each level has a certain amount of energy associated with it and the electrons can only jump levels if they gain or lose energy 4. Lowest energy levels closest to nucleus a. In the ____________________________ for an atom, electrons are at their lowest, most stable energy levels. ...
Atomic Structure and Chemical Bonding
... Ans2. Lyman series: When excited electrons in hydrogen atoms fall from higher energy levels to first energy level, the series of lines observed are called Lyman series. They are observed in ultraviolet region. Lyman = R(1/12 - 1/n2), n = 2, 3, 4, 5, ......... Balmer series: When excited electrons in ...
... Ans2. Lyman series: When excited electrons in hydrogen atoms fall from higher energy levels to first energy level, the series of lines observed are called Lyman series. They are observed in ultraviolet region. Lyman = R(1/12 - 1/n2), n = 2, 3, 4, 5, ......... Balmer series: When excited electrons in ...
schoa - Schieck
... 17. Compare atomic spectra to the continuous spectrum of light. 18. Why are atomic spectra compared to fingerprints? 19. Explain what is meant by the phrase “the energy of electrons in atoms in quantized”. 20. What successes did Bohr’s model of the atom have? 21. What failures did Bohr’s model have? ...
... 17. Compare atomic spectra to the continuous spectrum of light. 18. Why are atomic spectra compared to fingerprints? 19. Explain what is meant by the phrase “the energy of electrons in atoms in quantized”. 20. What successes did Bohr’s model of the atom have? 21. What failures did Bohr’s model have? ...
VIII. Other Types of Notations or Configurations
... VIII. Other Types of Notations or Configurations – 3. How is each sublevel filled? • A. Hund’s Rule – Every orbital in a subshell (s, p, d, f) is singly occupied with one e- before any one orbital is doubly occupied ...
... VIII. Other Types of Notations or Configurations – 3. How is each sublevel filled? • A. Hund’s Rule – Every orbital in a subshell (s, p, d, f) is singly occupied with one e- before any one orbital is doubly occupied ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.














![Chapter7_1 - Department of Chemistry [FSU]](http://s1.studyres.com/store/data/016128835_1-aea3c1aec04363d6cbf538e8faf80e45-300x300.png)