Polarity of Molecules
... Each F can accommodate an additional electron in its 2p subshell, but how can Be bind 2 fluorine atoms when it has no unpaired electrons? According to the VB model a bond results from sharing of unpaired electrons via overlap of AOs… ...
... Each F can accommodate an additional electron in its 2p subshell, but how can Be bind 2 fluorine atoms when it has no unpaired electrons? According to the VB model a bond results from sharing of unpaired electrons via overlap of AOs… ...
TERM 2 Unit 3 YR 9 SCI It is elementary
... 9 SCIENCE – Term 2 Unit 3: IT’S ELEMENTARY (5 WEEKS) ...
... 9 SCIENCE – Term 2 Unit 3: IT’S ELEMENTARY (5 WEEKS) ...
Slide1
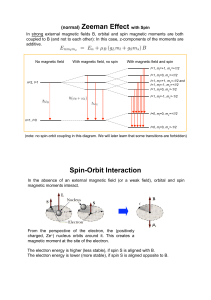
... Spin is like angular momentum Recall m can have (2l+1) values between –l and l. For spin, since only 2 ...
... Spin is like angular momentum Recall m can have (2l+1) values between –l and l. For spin, since only 2 ...
Midterm Study Guide with Answers
... OBJ: 5.1.1 Describe what Bohr proposed in his model of the atom. | 5.1.3 Explain how sublevels of principal energy levels differ. BLM: analysis 9. ANS: The aufbau principle states that electrons enter the orbitals of lowest energy first. The Pauli exclusion principle states that each orbital can hol ...
... OBJ: 5.1.1 Describe what Bohr proposed in his model of the atom. | 5.1.3 Explain how sublevels of principal energy levels differ. BLM: analysis 9. ANS: The aufbau principle states that electrons enter the orbitals of lowest energy first. The Pauli exclusion principle states that each orbital can hol ...
Atomic orbitals and their representation: Can 3-D
... Everywhere in space there is some finite probability for finding the particle. Electrons surrounding atoms are concentrated into regions of space assigned by atomic orbitals. The boundaries of an atomic orbital are conventionally drawn to the region of 90% probability, but they extend to infinity. F ...
... Everywhere in space there is some finite probability for finding the particle. Electrons surrounding atoms are concentrated into regions of space assigned by atomic orbitals. The boundaries of an atomic orbital are conventionally drawn to the region of 90% probability, but they extend to infinity. F ...
Details
... Structure of the atom, the early atomic theories, theories of electromagnetic radiation, Plank theory, Bohr theory. Quantum theory, Schrodinger wave equation, quantum numbers, shapes of orbitals and electronic configuration. ...
... Structure of the atom, the early atomic theories, theories of electromagnetic radiation, Plank theory, Bohr theory. Quantum theory, Schrodinger wave equation, quantum numbers, shapes of orbitals and electronic configuration. ...
Miss Pang`s 2012 Review
... 16. The atomic number of magnesium is 12. How many protons and valence electrons respectively does magnesium have? A) 12 and 12 ...
... 16. The atomic number of magnesium is 12. How many protons and valence electrons respectively does magnesium have? A) 12 and 12 ...
Thermochemistry (4 lectures)
... • Week 11: Terms and ionization energies Free-ion terms for d2 Ionization energies for 2p and 3d elements ...
... • Week 11: Terms and ionization energies Free-ion terms for d2 Ionization energies for 2p and 3d elements ...
Unit 3 Spiraling
... -There are only certain regions in the electron cloud where electrons are likely to be found. These regions are called energy levels. The lowest energy level is closest to the nucleus; the highest energy level is farthest away from the nucleus. Electrons will occupy the lowest available energy level ...
... -There are only certain regions in the electron cloud where electrons are likely to be found. These regions are called energy levels. The lowest energy level is closest to the nucleus; the highest energy level is farthest away from the nucleus. Electrons will occupy the lowest available energy level ...
Unit 3C Standards for Quiz
... Unit 2C Standards Quiz on Monday, November 24. It will be similar to the last exam but there will be at least three questions per standard. Remember that since no calculators are allowed on the standards exam that we will be modeling this in this assessment of progress. Atomic and Molecular Structur ...
... Unit 2C Standards Quiz on Monday, November 24. It will be similar to the last exam but there will be at least three questions per standard. Remember that since no calculators are allowed on the standards exam that we will be modeling this in this assessment of progress. Atomic and Molecular Structur ...
KS-DFT formalism
... has invaded our thinking is marked by our constant use of concepts which have meaning only in terms of independent particle wave functions: shell structure, the occupation number, the Fermi sea and the Fermi surface, the representation of perturbation theory by Feynman diagrams. All of these concept ...
... has invaded our thinking is marked by our constant use of concepts which have meaning only in terms of independent particle wave functions: shell structure, the occupation number, the Fermi sea and the Fermi surface, the representation of perturbation theory by Feynman diagrams. All of these concept ...
Final Exam 2004
... two atoms. For large R, the dipole-dipole interaction can be considered as a small perturbation. Show that the energy of the dipole-dipole interaction of the two atoms in their ground states is zero in the first order of the perturbation theory. [Hint: Since the ground state is nondegenerate, you ca ...
... two atoms. For large R, the dipole-dipole interaction can be considered as a small perturbation. Show that the energy of the dipole-dipole interaction of the two atoms in their ground states is zero in the first order of the perturbation theory. [Hint: Since the ground state is nondegenerate, you ca ...
Notes 12
... - it is generally difficult to solve the quantum mechanical problem of diatomic or polyatomic molecules with many electrons - frequently it is sufficient to consider the effect of the interaction between atoms on the outermost electron shell - electrons in the outermost shell are called valence elec ...
... - it is generally difficult to solve the quantum mechanical problem of diatomic or polyatomic molecules with many electrons - frequently it is sufficient to consider the effect of the interaction between atoms on the outermost electron shell - electrons in the outermost shell are called valence elec ...
Thursday, March 27, 2008
... A 100.00-gram sample of naturally occurring boron contains 19.78 grams of boron-10 (atomic mass = 10.01 atomic mass units) and 80.22 grams of boron-11 (atomic mass = 11.01 atomic mass units). Which numerical setup can be used to determine the atomic mass of naturally occurring boron? ...
... A 100.00-gram sample of naturally occurring boron contains 19.78 grams of boron-10 (atomic mass = 10.01 atomic mass units) and 80.22 grams of boron-11 (atomic mass = 11.01 atomic mass units). Which numerical setup can be used to determine the atomic mass of naturally occurring boron? ...
Total
... Direction: Write “T” in the bracket before the sentence, which you think to be correct, and “F” to be wrong. ...
... Direction: Write “T” in the bracket before the sentence, which you think to be correct, and “F” to be wrong. ...
chapter 6 sec 2 resonance structure
... H is 2.1 and O is 3.5. 3.5 – 2.1 = 1.4 so the bond between H and O is a polar covalent bond. By definition a neutral group of atoms held together by covalent bonds is a molecule. So, the H2O particle is a molecule H2O is a molecule which makes H2O a molecular compound and a molecular formula. But H2 ...
... H is 2.1 and O is 3.5. 3.5 – 2.1 = 1.4 so the bond between H and O is a polar covalent bond. By definition a neutral group of atoms held together by covalent bonds is a molecule. So, the H2O particle is a molecule H2O is a molecule which makes H2O a molecular compound and a molecular formula. But H2 ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.