Chemistry Notes
... and Neutrons. a) Proton – Positively charged particles. b) Neutron – Neutral particles. C. For the Elements, the number of electrons in an atom is equal to the number of Protons. This is called the Atomic Number. ...
... and Neutrons. a) Proton – Positively charged particles. b) Neutron – Neutral particles. C. For the Elements, the number of electrons in an atom is equal to the number of Protons. This is called the Atomic Number. ...
Matter and Energy Identify a chemical physical change Identify a
... Aufbau Principle Heisenberg Uncertainty Principle Hund’s rule Pauli exclusion Principle Ground and excited state Sublevels s p d f ...
... Aufbau Principle Heisenberg Uncertainty Principle Hund’s rule Pauli exclusion Principle Ground and excited state Sublevels s p d f ...
Ψ (x,t) = | Ψ (x,t) - University of Notre Dame
... Within the box, the energy is constant – hence ω and k are constant – and also the wavelength λ, and the momentum p Boundary conditions: The wave-function must go to zero outside the box, at very large positive and very large negative x-values Follow Bohr and De Broglie’s ideas and fix the number of ...
... Within the box, the energy is constant – hence ω and k are constant – and also the wavelength λ, and the momentum p Boundary conditions: The wave-function must go to zero outside the box, at very large positive and very large negative x-values Follow Bohr and De Broglie’s ideas and fix the number of ...
Arrangement of Electrons in Atoms
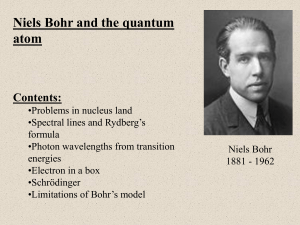
... From wavelengths of emission spectrum Bohr calculated energy levels of H-atom Model worked ONLY for H-atom ...
... From wavelengths of emission spectrum Bohr calculated energy levels of H-atom Model worked ONLY for H-atom ...
Document
... The work done in moving single electrical charges can be very small. Example: How much work is done in moving a 1.4 x 10-19 Coulomb electrical charge between two parallel plates with a potential difference of 10 Volts? ...
... The work done in moving single electrical charges can be very small. Example: How much work is done in moving a 1.4 x 10-19 Coulomb electrical charge between two parallel plates with a potential difference of 10 Volts? ...
ppt - HEP Educational Outreach
... •Each photon has two possible paths to reach a given point on the screen (e.g. - through Slit 1 or 2). •Quantum Mechanics says each path has an associated “probability amplitude”, which is a complex number that describes that path. •To determine the probability that a photon arrives at the point on ...
... •Each photon has two possible paths to reach a given point on the screen (e.g. - through Slit 1 or 2). •Quantum Mechanics says each path has an associated “probability amplitude”, which is a complex number that describes that path. •To determine the probability that a photon arrives at the point on ...
lecture 24
... more momentum than on shiny side-this is a bigger effect than the photon momentum ...
... more momentum than on shiny side-this is a bigger effect than the photon momentum ...
WS on obj. 1-11
... 3. _____ (T/F) The number of valence electrons is very important in determining the chemical properties of an element. 4. _____ (T/F) The elements of a group have different numbers of valence electrons. 5. _____ (T/F) The representative groups 1A-7A have the same number of valence electrons as their ...
... 3. _____ (T/F) The number of valence electrons is very important in determining the chemical properties of an element. 4. _____ (T/F) The elements of a group have different numbers of valence electrons. 5. _____ (T/F) The representative groups 1A-7A have the same number of valence electrons as their ...
Document
... • Anything that has volume and mass • Everything that exists is matter, and is made of ATOMS ...
... • Anything that has volume and mass • Everything that exists is matter, and is made of ATOMS ...
accelerate e
... 1942 magnetic induction -> Kerst build the betatron; 1944 synchrotron -> MacMillan, Oliphant and Veksel invent the RF phase stability (longitudinal focusing); 1946 proton linac -> Alvarez build an RF structure with drift tubes (progressive wave in 2p mode); 1950 strong focusing -> Christofilos paten ...
... 1942 magnetic induction -> Kerst build the betatron; 1944 synchrotron -> MacMillan, Oliphant and Veksel invent the RF phase stability (longitudinal focusing); 1946 proton linac -> Alvarez build an RF structure with drift tubes (progressive wave in 2p mode); 1950 strong focusing -> Christofilos paten ...
Chapter 5
... When = 1, there are three possible values of m (-1, 0, +1) and thus the porbital has three sub-orbitals. When = 2, there are five possible values of m (-2, -1, 0, +1, +2) and thus the d-orbital has five sub-orbitals. ...
... When = 1, there are three possible values of m (-1, 0, +1) and thus the porbital has three sub-orbitals. When = 2, there are five possible values of m (-2, -1, 0, +1, +2) and thus the d-orbital has five sub-orbitals. ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.