Document
... 1. A Feynman diagram consists of external lines (lines which enter or leave the diagram) and internal lines (lines start and end in the diagram). External lines represent physical particles (observable). Internal lines represent virtual particles ( A virtual particle is just like a physical particle ...
... 1. A Feynman diagram consists of external lines (lines which enter or leave the diagram) and internal lines (lines start and end in the diagram). External lines represent physical particles (observable). Internal lines represent virtual particles ( A virtual particle is just like a physical particle ...
Ch - cmpascience
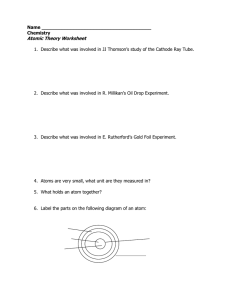
... 1. Explain Dalton’s atomic theory, and describe why it was more successful than Democritus’s theory. 2. State the charge, mass, and location of each part of an atom according to the modern model of the atom. 3. Compare and contrast Bohr’s model with the modern model of an atom. Dalton’s Theory 1. Ev ...
... 1. Explain Dalton’s atomic theory, and describe why it was more successful than Democritus’s theory. 2. State the charge, mass, and location of each part of an atom according to the modern model of the atom. 3. Compare and contrast Bohr’s model with the modern model of an atom. Dalton’s Theory 1. Ev ...
May 2001
... In this problem, we investigate the effect of electromagnetic waves traveling through a gas of charged particles. This can happen when there is radio emission from a pulsar, and these signals propagate through clouds of charged particles in deep space before being detected on Earth. A linearly polar ...
... In this problem, we investigate the effect of electromagnetic waves traveling through a gas of charged particles. This can happen when there is radio emission from a pulsar, and these signals propagate through clouds of charged particles in deep space before being detected on Earth. A linearly polar ...
Charging of positively charged dust particle in weak magnetic field*
... magnetic field was expressed by the OML (Orbit Motion Limited) theory [1, 2]. The OML theory, where energy and angular momentum of a charged particle are conserved in an infinite Debye length limit, has been widely applied to charging of a dust particle in space plasmas as well as laboratory plasmas ...
... magnetic field was expressed by the OML (Orbit Motion Limited) theory [1, 2]. The OML theory, where energy and angular momentum of a charged particle are conserved in an infinite Debye length limit, has been widely applied to charging of a dust particle in space plasmas as well as laboratory plasmas ...
Supercomputing in High Energy Physics
... Big Bang. It also explores physics at temperatures not common for the past 15 billion years (or so). ...
... Big Bang. It also explores physics at temperatures not common for the past 15 billion years (or so). ...
UQ3
... 2. Cesium-137 radioactively decays via beta particle decay. Write the nuclear equation for this beta particle decay process and identify the nuclide produced. ...
... 2. Cesium-137 radioactively decays via beta particle decay. Write the nuclear equation for this beta particle decay process and identify the nuclide produced. ...
Physics 12 Assignment - hrsbstaff.ednet.ns.ca
... Lyman series Balmer series Paschen series quantum number Bohr radius ground state excited states binding energy (or ionization energy) 2. In Rutherford’s planetary model of the atom, what keeps the electrons from flying off into space? 3. How can the spectrum of hydrogen contain so m ...
... Lyman series Balmer series Paschen series quantum number Bohr radius ground state excited states binding energy (or ionization energy) 2. In Rutherford’s planetary model of the atom, what keeps the electrons from flying off into space? 3. How can the spectrum of hydrogen contain so m ...
lecture 15 (zipped power point) (update: 2 Jan 03)
... What is its wavelength? Answer: 0.51 MeV, annih = hc / 0.51 MeV = 0.0243 nm The detection of such characteristic gamma ray in astrophysics indicates the annihilation of matterantimatter in deep space May indicate the existence of ‘anti-matter world’ However, none of this is observed ...
... What is its wavelength? Answer: 0.51 MeV, annih = hc / 0.51 MeV = 0.0243 nm The detection of such characteristic gamma ray in astrophysics indicates the annihilation of matterantimatter in deep space May indicate the existence of ‘anti-matter world’ However, none of this is observed ...
de broglie waves - Project PHYSNET
... totally alien to the picture of two colliding particles. Yet, as we have seen, the same “particle” really can exhibit both wave and particle aspects. The resolution of this seeming paradox requires that we give up trying to learn the exact positions of particles, and give up thinking of waves as “ph ...
... totally alien to the picture of two colliding particles. Yet, as we have seen, the same “particle” really can exhibit both wave and particle aspects. The resolution of this seeming paradox requires that we give up trying to learn the exact positions of particles, and give up thinking of waves as “ph ...
Chem 1st Sem Rev Ch2
... 1. Arrange these elements in order of decreasing atomic size: sulfur, chlorine, aluminum and sodium. Does your arrangement demonstrate a periodic or group trend? 2. Distinguish between the first and second ionization energies of an atom. 3. Indicate which element in each of the following pairs has t ...
... 1. Arrange these elements in order of decreasing atomic size: sulfur, chlorine, aluminum and sodium. Does your arrangement demonstrate a periodic or group trend? 2. Distinguish between the first and second ionization energies of an atom. 3. Indicate which element in each of the following pairs has t ...
Atomic Spectra
... E RH 2 2 nl nh where RH is the Rydberg constant for hydrogen (= 2.179 × 10-18 J = 13.61 eV = 109677 cm-1); nl nh , are integers (l for lower lever and h for higher lever). ...
... E RH 2 2 nl nh where RH is the Rydberg constant for hydrogen (= 2.179 × 10-18 J = 13.61 eV = 109677 cm-1); nl nh , are integers (l for lower lever and h for higher lever). ...
CHEM 347 Quantum Chemistry
... h is a constant of proportionality ν radiation’s frequency In other words, Planck proposed that the energy of the oscillators (the material!) was quantized and that only certain quantities of light energy could be emitted. ...
... h is a constant of proportionality ν radiation’s frequency In other words, Planck proposed that the energy of the oscillators (the material!) was quantized and that only certain quantities of light energy could be emitted. ...
$doc.title
... Now consider a quantum state with two particles: Suppose we have two quantum states a(x) and b(x) each with a distinguishable particle in it. For example we might have an electron in state a and a proton in state b. Now the probability of finding the electron in position x1 in state a is |a(x1)|2 ...
... Now consider a quantum state with two particles: Suppose we have two quantum states a(x) and b(x) each with a distinguishable particle in it. For example we might have an electron in state a and a proton in state b. Now the probability of finding the electron in position x1 in state a is |a(x1)|2 ...
Glencoe Chapter 4 Structure of the Atom for the Wiki
... in the same ratio by mass. Law of Multiple Proportions Based on atomic theory but no experiment evidence at the time • The ratio of the masses of one element that combine with a constant mass of another element can be expressed in small whole numbers. ...
... in the same ratio by mass. Law of Multiple Proportions Based on atomic theory but no experiment evidence at the time • The ratio of the masses of one element that combine with a constant mass of another element can be expressed in small whole numbers. ...
AtomLightEmissQuantum
... What would you expect to see if you viewed the glowing filament through a diffraction grating? When viewed in this way, all of the colors of the rainbow would be visible. The bulb also emits infrared radiation that you would not see. A plot of the intensity of the light emitted from a hot body over ...
... What would you expect to see if you viewed the glowing filament through a diffraction grating? When viewed in this way, all of the colors of the rainbow would be visible. The bulb also emits infrared radiation that you would not see. A plot of the intensity of the light emitted from a hot body over ...
N - MPS
... The neutral collision frequency, n, i.e. number of collisions per second, is proportional to the number of neutral particles in a column with a cross section of an atom or molecule, nnn, where nn is the density and n= d02 ( 10-20 m2) the atomic cross section, and to the average speed, < > ( ...
... The neutral collision frequency, n, i.e. number of collisions per second, is proportional to the number of neutral particles in a column with a cross section of an atom or molecule, nnn, where nn is the density and n= d02 ( 10-20 m2) the atomic cross section, and to the average speed, < > ( ...
1 - Lagan Physics
... 5. Draw Feynman diagrams and explain what happens in (a) beta-minus decay; (b) positron decay & (c) electron capture. 6. Try the summary questions on page 15 ...
... 5. Draw Feynman diagrams and explain what happens in (a) beta-minus decay; (b) positron decay & (c) electron capture. 6. Try the summary questions on page 15 ...
Quantum mechanic and Particle physics
... • Plank, 1931: “By then I had been wrestling unsuccessfully for six years with the problem … and I knew it was of fundamental importance to physics… A theoretical interpretation therefore had to be found at all costs, no matter how high… The new approach was opened to me by maintaining the two law ...
... • Plank, 1931: “By then I had been wrestling unsuccessfully for six years with the problem … and I knew it was of fundamental importance to physics… A theoretical interpretation therefore had to be found at all costs, no matter how high… The new approach was opened to me by maintaining the two law ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.