* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Levetiracetam in the Treatment of Epilepsy
Aging brain wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Long-term depression wikipedia , lookup
Multielectrode array wikipedia , lookup
History of neuroimaging wikipedia , lookup
Signal transduction wikipedia , lookup
Neuroplasticity wikipedia , lookup
Nervous system network models wikipedia , lookup
Neuroeconomics wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Neural oscillation wikipedia , lookup
NMDA receptor wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Neural engineering wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Neuroregeneration wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Subventricular zone wikipedia , lookup
Apical dendrite wikipedia , lookup
Transcranial direct-current stimulation wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Development of the nervous system wikipedia , lookup
Synaptic gating wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Microneurography wikipedia , lookup
Neuroanatomy wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Synaptogenesis wikipedia , lookup
Haemodynamic response wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Neurostimulation wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Metastability in the brain wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Optogenetics wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Temporal lobe epilepsy wikipedia , lookup
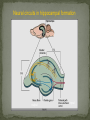
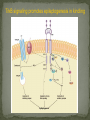
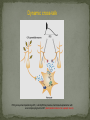
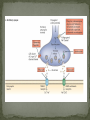
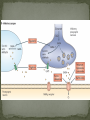
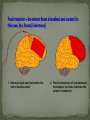
Epilepsy Yung-Yang Lin (林永煬), MD, PhD National Yang-Ming University Taipei Veterans General Hospital Outline Epidemiology Diagnosis Etiologies and Mechanisms Treatment Epidemiology The incidence is around 50/100 000/year. Prevalence of active epilepsy is in the range of 5-10/1000. Age-specific incidence rates: a decrease in younger age groups and an increase in persons above 60 years Overall prognosis for seizure control is good and over 70% will enter remission. Increased risk of premature death particularly in patients with chronic epilepsy (Sudden unexpected death ) Diagnosis History of event Medical history Blood tests Electroencephalography (EEG) Simultaneous EEG and video recordings Brain scanning (CT scan, MRI) - to discover if the patient has symptomatic epilepsy; a structural cause for their seizures PET, SPECT, MRS Magnetoencephalography (MEG) Etiologies and Mechanisms Degenerative brain disorder 3.5% Infection 2.5% Neoplasm 4.1% Idiopathic and cryptogenic epilepsy 65.5% Vascular injury 10.9% Trauma 5.5% Congenital causes 8.0% 100 90 80 70 60 50 40 30 20 10 0 Others Degenerative Cerebrovascular Brain tumour Trauma Infection Development 0–4 5–14 15–24 25–44 45–64 65+ In rare cases patients may have one specific trigger that brings on a seizure, for example: Flashing visual stimuli Looking at a particular kind of pattern Hearing a particular piece of music Reading In the majority of cases an epileptic seizure ends of its own accord Status epilepticus is a condition characterized by an epileptic seizure that is so frequently repeated or prolonged as to create a fixed and lasting condition It is a medical emergency that requires prompt and appropriate treatment An abnormal synchronous and sustained activity (overexcitation) in a group of nerve cells This group of nerve cells = epileptogenic focus Abnormal interictal activity When this focus recruits surrounding, normal nerve cells into a synchronous pattern of larger abnormal activity (burst firing), there is transition from interictal to ictal activity = SEIZURE Excess excitation Lack of inhibition epileptic seizures epileptic seizures Hippocampal sclerosis (1) Extensive neuronal loss and gliosis in the areas of CA1 and the hilus but also in other hippocampal regions to varying degrees. (2) Synaptic reorganization, although not necessarily limited to the mossy fibers of the dentate gyrus. (3) Dispersion of the dentate granule cells. (4) Extrahippocampal pathology (i.e.neuronal loss in the neighboring entorhinal cortex and amygdala). Neural circuits in hippocampal formation output input Hilar neuronal loss and mossy fiber sprouting Sprouting is classically seen as a response to the loss of neuronal targets: the loss of mossy cells and somatostatin-positive interneurons in the hilus lead to mossy fiber sprouting in the inner and outer molecular layers. Mossy fibers in humans with MTLE and in animal MTLE models: form excitatory recurrent circuits through collaterals synapsing onto granule cell and interneuron dendrites in the supragranular layer and onto new subgranular dendrites in the hilus. Molecular mechanisms underlying epileptogenesis NMDA receptor activation Group I mGluR activation in CA3 pyramidal neurons TrkB signaling Cross-talk between neurons and astrocytes (synchronous epileptiform activity in CA1 pyramidal neurons) Activation of NMDA receptors (at postsynaptic sites on dendritic spines) Ca2+ influx CaMKII and calcineurin activation CaMKII calcineurin GluR1 of AMPA receptors internalization of GABAA receptor [Ca2+]i GABA-mediated synaptic inhibition Ca2+-dependent gene expression Mossy fiber sprouting Epileptogenesis KCC2 TrkB signaling promotes epileptogenesis in kindling Astrogliosis – abnormal shape and increased numbers of astrocytes – is a prominent feature of Ammon’s horn sclerosis. Glu released from neurons can activate mGluR on astrocytes. Glu released from an astrocyte is sufficient to trigger a PDS (paroxysmal depolarizing shift) in neighboring neuron. A novel mechanism for the synchronization of neuronal firing Positive feedback model Dynamic cross-talk PDS (paroxysmal depolarizing shift) : a brief(250ms) massive membrane depolarization with an accompanying burst of AP. (best cellular marker of an epileptic event) Treatment Treatment of underlying causes Trigger avoidance Drug therapy Surgery Ketogenic diet Vagus nerve stimulation Deep brain stimulation Complementary therapies Medications and action mechanisms Selection of antiepileptic drugs (AEDs) based on: ‘Standard’ vs ‘new’ drug Spectrum of efficacy Tolerability Pharmacokinetics Mode of action Standard Phenytoin (PHT), Pfizer (Dilantin) Carbamazepine (CBZ), Novartis (Tegretol) Sodium valproate (VPA), Sanofi Synthelabo (Depakine) Ethosuximide (ESM), Pfizer Barbiturates Phenobarbital (PB) Primidone (PRM) New Felbamate (FBM), Carter-Wallace Vigabatrin (VGB), Aventis(Sabril) Lamotrigine (LTG), GSK(Lamictal) Gabapentin (GBP), Pfizer(Neurontin) Topiramate (TPM), Janssen-Cilag(Topamax) Tiagabine (TGB), Sanofi Synthelabo(Gabatril) Oxcarbazepine (OCBZ), Novartis(Trileptal) Benzodiazepines Clonazepam (CZP) Clobazam (CLB) Zonisamide (ZNS), Athena Decreased excitation – via blockade of sodium channels, interaction with voltage-sensitive calcium channels or blockade of glutamate receptors. Increased inhibition – via an increase in the concentration of GABA in the synaptic cleft. First drug Success rate 50% Failure rate 50% Alternative monotherapy Success rate 20% Failure rate 30% Dual therapy Success rate 5% Failure rate 25% 1. Sub-dural grid used to localise the site of seizure onset 2. Frontal lobectomy of non-dominant hemisphere (red area indicates the extent of resection) Vagus nerve stimulation Vagus nerve stimulation Alteration of norepinephrine release by projections of solitary tract to the locus coeruleus Elevated levels of inhibitory GABA related to vagal stimulation Inhibition of aberrant cortical activity by reticular system activation Deep brain stimulation Deep brain stimulation Probably mimics that of high frequency DBS for movement disorders Neurons adjacent to stimulating electrodes appear to undergo long term inactivation following stimulation, leading to interruption of pathologic network activity