* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Carl L.Faingold, Manish Raisinghani, Prosper N`Gouemo
Biological neuron model wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Types of artificial neural networks wikipedia , lookup
Aging brain wikipedia , lookup
Mirror neuron wikipedia , lookup
Convolutional neural network wikipedia , lookup
Perception of infrasound wikipedia , lookup
Adult neurogenesis wikipedia , lookup
Neural oscillation wikipedia , lookup
Development of the nervous system wikipedia , lookup
Multielectrode array wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Metastability in the brain wikipedia , lookup
Neuroanatomy wikipedia , lookup
Circumventricular organs wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Sexually dimorphic nucleus wikipedia , lookup
Psychophysics wikipedia , lookup
Neural coding wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Nervous system network models wikipedia , lookup
Central pattern generator wikipedia , lookup
Substantia nigra wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Synaptic gating wikipedia , lookup
Optogenetics wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
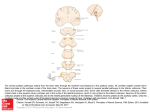
Chapter 26 Neuronal Networks in Epilepsy Comparative Audiogenic Seizure Networks Carl L.Faingold, Manish Raisinghani, Prosper N'Gouemo Copyright © 2014 Elsevier Inc. All rights reserved. 1 FIGURE 26.1 Comparative network diagrams in three forms of audiogenic seizures: GEPR-9s (left panel), GEPR-3s (middle panel), and ETX rats (right panel). The required network structures in all three models begins in the auditory network [the cochlear nucleus (CN) and superior olivary complex (SOC)], which project to the critical hub in the inferior colliculus (IC). The IC projects to the deep layers of the superior colliculus (DLSC) and brainstem conditional multireceptive regions, including the (pontine) brainstem reticular formation (BRF) and ventrolateral periaqueductal gray (PAG) and substantia nigra reticulata (SNr). These structures project to the spinal cord, which generates the wildrunning and tonic seizures, which end in tonic extension of the hind limbs in GEPR-9s and which end in forelimb clonus in GEPR-3s and ETX rats (with some variability). However, in the GEPR-3s and ETX rats, the amygdala (AMG) is also involved in the network, and the medial geniculate body (MGB) is implicated, since this structure is the likely pathway from the IC to the AMG. The hippocampus (HPC) is implicated in the network for ETX. The cortex is not involved in either GEPR-9s or GEPR-3s unless further intervention occurs (see Chapter 27). Copyright © 2014 Elsevier Inc. All rights reserved. 2 FIGURE 26.2 Composite behavioral activity in GEPR-9s and concurrent typical examples of neuronal firing in the major network nuclei for AGS seen during each seizure behavior. The structures include the (A) inferior colliculus central (ICc) nucleus; (B) inferior colliculus external (ICx) nucleus; (C) deep layers of the superior colliculus (DLSC); (D) periaqueductal gray (PAG); (E) pontine brainstem reticular formation (PRF); and (F) substantia nigra reticulata (SNr). Thus, ICc and ICx firing is greatest during AGS initiation, while the greatest increase in DLSC neuronal firing occurs at the onset of wild running. The greatest increases in PAG, SNr, and PRF neuronal firing occur during the tonus and tonic hindlimb extension phases of AGS. The PRF and SNr are the only areas active during postictal depression of consciousness. Note: Recovery of SNr firing occurred but was not recorded in the example in (F). Acoustic stimulus parameters: 12 kHz tone burst, 100 ms duration, 5 ms rise-fall, 100 dB SPL, 2–4 Hz repetition rate. Each row is from the same rat. (Source: Drawings courtesy of Naritoku et al. From Faingold by permission.) Copyright © 2014 Elsevier Inc. All rights reserved. 3 FIGURE 26.3 GABA-mediated inhibition defects in GEPR-9s: GABAergic neurotransmission normally plays a critical role in determining the responses of the inferior colliculus (IC) to acoustic stimulation, and defects in specific forms of inhibition are key causative factors in audiogenic seizure initiation. Line (A) illustrates binaural inhibition common in ICc neurons. In the poststimulus time histogram (PSTH) example in line (A) (“Normal” column), binaural presentation of the stimulus results in a greatly reduced number of action potentials (N), as compared to the response to the same stimulus to one ear (monaurally to the contralateral ear). In GEPR-9 ICc neurons, the effect of binaural inhibition is significantly reduced, as shown in this example by the comparison of the line (A) (“GEPR-9” column) example when the sound is presented to both ears (binaural). This difference in effectiveness of GABA-mediated inhibition was significant, as shown by the bar graphs in line (A), illustrating the mean normal versus GEPR IC data. This form of inhibition can be blocked by direct application of a GABAA antagonist onto ICc neurons.151 Line (B) illustrates examples of high-intensity-induced inhibition (a nonmonotonic rate intensity response), which is a form of inhibition seen in many normal ICc neurons when the intensity of the acoustic stimulus is increased, as shown by the rate–intensity curve in line (B) (“Normal” column), and this inhibition can be blocked by application of a GABAA antagonist, bicuculline (BIC), directly onto these neurons, as also shown by the example in 3B (“Normal” column). An example of the failure of this form of inhibition in the GEPR is shown in line (B) (“GEPR-9” column), and the significant mean difference in the normal versus GEPR comparison is shown by the bar graph in line (B). A form of inhibition that occurs at the end of a finite acoustic stimulus is called the offset response and is seen in a small percentage of normal ICc neurons, but it is often hard to detect [line (C), “Control” column]. However, when BIC is applied directly onto many ICc neurons, this can cause an offset peak to appear by blocking offset inhibition [line (C), “Normal” column, right panel]. In GEPR-9s, the incidence of failure of offset inhibition allowing the appearance of the offset response are often induced by increasing the sound intensity [line (C), “GEPR-9” column, right panel]. The incidence of neurons showing the offset peak was significantly greater in GEPR-9s as compared to normal rats, as shown by the bar graphs in line (C). Direct iontophoretic application of GABA onto ICc neurons produces significant inhibition in acoustically evoked firing [line (D), “Normal” panel, right versus left panel at 2 nA of GABA]. In GEPR-9s, the effectiveness of iontophoretically applied GABA was significantly reduced [line (D), “GEPR-9” column, right versus left panel, 20 nA of GABA], despite the fact that this was applied using the same electrode in both rats. The mean difference in GABA effectiveness was significant, as shown by the bar graphs in line (D). PSTH parameters = 25 or 50 stimulus presentations at 2/s of 100 ms 12 kHz tone bursts (100 ms duration denoted by the heavy line beneath each PSTH; 5 ms rise-fall). N = number of action potentials per PSTH. ∗ Significantly different at p < 0.05; # significantly different at p < 0.02. Copyright © 2014 Elsevier Inc. All rights reserved. 4 FIGURE 26.4 In animals susceptible to AGS (in GEPRs and during ETX), paired-pulse inhibition in inferior colliculus dorsal nucleus (ICd) neurons (in vitro) is replaced by facilitation as compared to responses in normal ICd neurons. Paired-pulse inhibition was seen in normal (Norm) ICd neurons [(D) low intensity; (E) moderate intensity; (F) high intensity] in response to increasing stimulus intensity in the IC commissure. In GEPRs, paired-pulse facilitation was observed [(A) low intensity; (B) moderate intensity; (C) high intensity]. A similar effect was seen in ICd neurons during ETX, as shown by the significant differences in the mean incidence of facilitation shown in the bar graphs in the right panel. (Source: Based on Refs 152,153.) ∗ p < 0.05: Significantly different from normal controls (Student's t-test). (Source: Reprinted from Faingold,30 with permission.) Copyright © 2014 Elsevier Inc. All rights reserved. 5 FIGURE 26.5 Compilation of mean acoustically evoked neuronal firing in GEPR-9s as a percentage, with normal Sprague–Dawley rats as control (100%). The acoustic stimuli (12 kHz 100 ms tone burst) was at 90 dB (SPL Re 0.0002 dyn/cm2). Five AGS network sites are compared: the inferior colliculus (IC), deep layers of the superior colliculus (DLSC), periaqueductal gray (PAG), pontine reticular formation (PRF), and substantia nigra reticulata (SNr). The mean neuronal firing percentages were increased in all the network structures with the greatest increase in the consensus AGS initiation site in the IC. Significantly different from control at ∗ p < 0.05 (Student's t-test). Copyright © 2014 Elsevier Inc. All rights reserved. 6 FIGURE 26.6 Compilation of mean acoustic response threshold differences between GEPR-9s and normal Sprague– Dawley rats. Five AGS network sites are compared: the inferior colliculus (IC), deep layers of the superior colliculus (DLSC), periaqueductal gray (PAG), pontine reticular formation (PRF), and substantia nigra reticulata (SNr). In the IC, DLSC, and PAG, the response thresholds are significantly above those of normal rats, consistent with the known hearing deficit in these rats. However, in the PRF and SNr, the neuronal response threshold is significantly below that of the neurons in the same structures in normal rats. Significantly different from control at ∗ p < 0.05 and ∗∗ p < 0.01 (Student's t-test). Copyright © 2014 Elsevier Inc. All rights reserved. 7