* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 8
Ultrasensitivity wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Citric acid cycle wikipedia , lookup
Point mutation wikipedia , lookup
Restriction enzyme wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Peptide synthesis wikipedia , lookup
Interactome wikipedia , lookup
Genetic code wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Western blot wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Protein structure prediction wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Catalytic triad wikipedia , lookup
Metalloprotein wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Proteolysis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Biosynthesis wikipedia , lookup
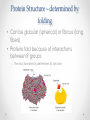
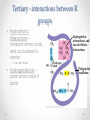
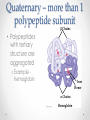
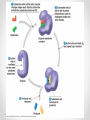
Chapter 3 Proteins and Enzymes (Chapter 7) Protein Structure – determined by folding • Can be globular (spherical) or fibrous (long fibers) • Proteins fold because of interactions between R groups o The structure directly determines its function Four levels of protein structure • Primary – sequence of amino acids • Secondary – coils or folds in backbone • Tertiary – interactions between R groups • Quaternary – more than 1 polypeptide subunit Fig. 5-21a Primary Structure 1 5 H3N Amino end + 10 Amino acid subunits 15 20 25 Secondary – coils or folds in backbone • H bonds between the backbone (not the R groups) • helix – a coil • pleated sheet – folded structure Tertiary - interactions between R groups • Hydrophobic interactions – nonpolar amino acids end up clustered in center o Van der Waals • Hydrogen Bonds – polar amino acids H bond Hydrophobic interactions and van der Waals interactions Hydrogen bond Polypeptide backbone Tertiary - interactions between R groups • Ionic Bonds – positively and negatively charged aas bond • Disulfide bridges – covalent bonds of two cysteines (–SH) Polypeptide backbone Disulfide bridge Ionic bond Quaternary – more than 1 polypeptide subunit β Chains • Polypeptides with tertiary structure are aggregated o Example hemoglobin Iron Heme α Chains Hemoglobin Denaturation - unraveling • Caused by: o High or low pH o Increased salt concentration o High temps Enzymes • catalytic proteins • speed up metabolic reactions by lowering energy barriers • not consumed or changed Enzymes • substrate – the reactant that an enzyme acts on • enzyme-substrate complex: the enzyme bound to its substrate • active site – the region on the enzyme where the substrate binds • induced fit – the way the substrate fits into the active site (the enzyme changes shape slightly) Activation Energy (EA) • needed to start a chemical reaction o heat from the surroundings Enzymes Lower the EA Barrier Enzyme Activity • Lower an EA barrier by: o Orienting substrates correctly o Straining substrate bonds o Providing a favorable microenvironment o Covalently bonding to the substrate Enzyme Activity • Affected by: o temperature o pH o chemicals that specifically influence the enzyme Enzymes and Biotechnology • Human babies are born with lactase, an enzyme that breaks down lactose found in milk • Naturally, as humans age they become increasingly lactose intolerant as they stop producing lactase • Some cultures have higher percentages of people who are lactose intolerant (Asia), some lower percentages (Europe) • Lactase is produced on large scales to create lactosefree products Enzymes and Biotechnology Enzyme Inhibitors Competitive inhibitors • Bind to the active site • Compete with the substrate Competitive Example: • Ethylene glycol: antifreeze o Ethanol is used to treat ethylene glycol poisoning o Reversible inhibition Ethylene glycol ethanol (inhibitor) Products formed Cause irreversible damage to the kidneys Competitive Example: • Sulfanilamide: antibiotic o similar structure to paraaminobenzoic acid (PABA), (pathway for folic acid) o IRREVERSIBLE COMPETITIVE INHIBITION PABA Sulphanilamide (inhibitor) Folic acid NO Folic acid formation after inhibition Enzyme Inhibitors Noncompetitive inhibitors • Bind to another part of an enzyme • Cause the enzyme (active site) to change shape Noncompetitive Example: Morphine • Binds to a site other than the active site of the enzyme Nitric oxide synthase Arginine • The enzyme stays inhibited Nitric oxide synthase Citrulline Nitric oxide Noncompetitive Example: Cyanide (CN-) • Attaches to the –SH group of an enzyme CYTOCHROME C OXIDASE • results in the inhibition of cellular respiration!!! Cytochrome c oxidase OXYGEN WATER Feedback Inhibition • The end product of a metabolic pathway shuts down the pathway • Prevents wasting resources