* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lecture 8: The cell membrane
Survey
Document related concepts
Extracellular matrix wikipedia , lookup
Mechanosensitive channels wikipedia , lookup
Cell nucleus wikipedia , lookup
Magnesium transporter wikipedia , lookup
Cytokinesis wikipedia , lookup
Membrane potential wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Theories of general anaesthetic action wikipedia , lookup
SNARE (protein) wikipedia , lookup
Signal transduction wikipedia , lookup
Lipid bilayer wikipedia , lookup
Ethanol-induced non-lamellar phases in phospholipids wikipedia , lookup
Model lipid bilayer wikipedia , lookup
Cell membrane wikipedia , lookup
Transcript
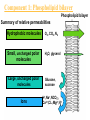
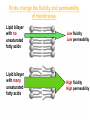
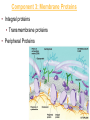
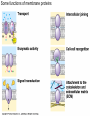
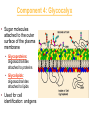
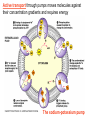
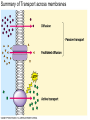
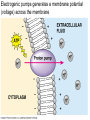
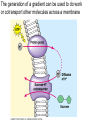
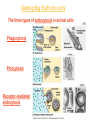
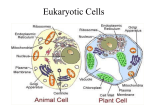
Membranes • A cell’s plasma membrane defines a cell and separates it from its environment. Yet at the same time, it must allow for interaction between the cell and its environment. Simple Membranes and the First Cells Biological Evolution began with the first selfreplicating molecule The first cell arose when this molecule became enclosed in a membrane CHEMICAL EVOLUTION Light energy Heat H H C O 1. Simple molecules in atmosphere of ancient Earth 2. Reduced carboncontaining compounds H H HOCH2 O OH C H H C H C C O H C C H H HOOH 3. First carboncarbon bonds Membranes are Composed of Phospholipids • Amphipathic lipids can form a bilayer structure in an aqueous solution One phospholipid: Phosphate group (hyd rophilic) Glyce rol Fatty Acids (hyd rophobi c) Lipid Bilay er: Liposomes: Artificial membranebound vesicles Water Water 62.5 nm Liposomes will form spontaneously in solution The Fluid Mosaic model of Plasma Membranes • Phospholipid Bilayer • Cholesterol • Glycocalyx • Proteins Evidence for the drifting of membrane proteins Component 1: Phospholipid bilayer Phospholipid bilayer Summary of relative permeabilities Hydrophobic molecules O2, CO2, N2 Small, uncharged polar molecules H2O, glycerol Large, uncharged polar molecules Ions Glucose, sucrose H+,Na+,NCO3–, Ca2+,CL-,Mg2+,K+ Phospholipids may have saturated or unsaturated fatty acid tails Double bonds cause H C kinks in hydrocarbons. 2 CH2 H2C CH2 H2C CH Kink CH H2C H2C H2C CH2 CH2 Unsaturated fatty acid Saturated fatty acid Double bonds present, fewer H atoms No Double bond, maximum H atoms Kinks change the fluidity and permeability of membranes. Lipid bilayer with no unsaturated fatty acids Low fluidity Low permeability Lipid bilayer with many unsaturated fatty acids High fluidity High permeability Component 2: Cholesterol fills spaces between phospholipids. Polar Nonpolar The more cholesterol within the membrane, the less permeable it is Cholesterol keeps the membrane fluid at low temps, prevents it from becoming too fluid at warmer temps Component 3: Membrane Proteins • Integral proteins • Transmembrane proteins • Peripheral Proteins Transmembrane proteins are amphpathic Glu Polar amino acids Tyl Met Pro Ile Pro Gly Ser Asp Non- Polar amino acids Some functions of membrane proteins Component 4: Glycocalyx • Sugar molecules attached to the outer surface of the plasma membrane • Glycoproteins: oligosaccharides attached to proteins • Glycolipids: oligosaccharides attached to lipids • Used for cell identification: antigens Movement of Substances Across Membranes • Diffusion • Molecules will diffuse from areas of high concentration to areas of lower concentration • Diffusion is a spontaneous, passive process (doesn’t require the input of external energy) . Each type of molecule will travel down its concentration gradient across a permeable membrane Water will move down (diffuse) its own concentration gradient--> osmosis solvent solute water is also moving in the direction of lower solute concentration to higher solute concentration Water enters vesicle if internal solution is hypertonic to the external solution. Start with: Hypertonic solution Hypotonic solution Isotonic solution Arrows represent direction that water moves via osmosis Result: Membrane shrinks Membrane swells or even bursts No change Figure 8.12 The water balance of living cells Movement of Substances Across Membranes • Movement across membranes is affected by the presence of membrane proteins. • 3 types of transporter proteins: • Channel Proteins • Carriers • Pumps Facilitated diffusion follows the concentration gradient and requires no input of external energy Channel Protein Carrier Protein Active transport through pumps moves molecules against their concentration gradients and requires energy The sodium-potassium pump Summary of Transport across membranes Electrogenic pumps generates a membrane potential (voltage) across the membrane The generation of a gradient can be used to do work or cotransport other molecules across a membrane Getting Big Stuff Out of Cells Exocytosis Getting Big Stuff into cells The three types of endocytosis in animal cells Phagocytosis Pinocytosis Receptor-mediated endocytosis When transport doesn’t work: Cystic Fibrosis • Cystic fibrosis is a defect in the Cl- transporter, CFTR • About 1 baby in 2000 of Northern European racial origin is affected • Symptoms • thick, sticky mucus that clogs the lungs and leads to lung infections. • Obstruction of the pancreas, preventing digestive enzymes from reaching the intestines