* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Projection patterns from the amygdaloid nuclear complex to
Neuroeconomics wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Development of the nervous system wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Metastability in the brain wikipedia , lookup
Axon guidance wikipedia , lookup
Neuroplasticity wikipedia , lookup
Central pattern generator wikipedia , lookup
Aging brain wikipedia , lookup
Limbic system wikipedia , lookup
Neuroanatomy wikipedia , lookup
Neuroanatomy of memory wikipedia , lookup
Orbitofrontal cortex wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Basal ganglia wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Optogenetics wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
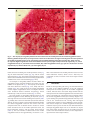
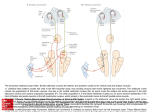
BR A I N R ES E A RC H 1 1 4 3 ( 2 00 7 ) 1 1 6 –12 5 a v a i l a b l e a t w w w. s c i e n c e d i r e c t . c o m w w w. e l s e v i e r. c o m / l o c a t e / b r a i n r e s Research Report Projection patterns from the amygdaloid nuclear complex to subdivisions of the dorsal raphe nucleus in the rat Hyun S. Leea,⁎, Yun J. Euma , Seung M. Job , Barry D. Waterhouse c a Department of Anatomy, College of Medicine, Konkuk University, Chungju, Chungbuk 380-701, South Korea Department of Anatomy, College of Medicine, Gachon University, Incheon, Kyunggi 405-220, South Korea c Department of Neurobiology and Anatomy, College of Medicine, Drexel University, 2900 Queen Lane, Philadelphia, PA 19129, USA b A R T I C LE I N FO AB S T R A C T Article history: The goal of the present study was to identify the projection from the subdivisions of the Accepted 17 January 2007 amygdaloid nuclear complex to specified subregions of the dorsal raphe (DR) nucleus and to Available online 28 January 2007 attempt to compare the density of amygdaloid input to the DR with that of inputs from other limbic structures. Use of a retrograde tracer, gold-conjugated and inactivated wheatgerm Keywords: agglutinin-horseradish peroxidase (WGA-apo-HRP-gold), demonstrated that amygdaloid Amygdala input to midline DR subdivision originates mainly from the medial portion of the medial Dorsal raphe amygdaloid nucleus, whereas that to lateral wing subdivision derives from the region Tract tracing extending from the lateral portion of the medial amygdaloid nucleus to the commissural Fluorogold stria terminalis. Use of the retrograde tracer Fluorogold (FG) produced relatively large but WGA-apo-HRP-gold circumscribed injection sites comprising midline DR as well as portions of lateral wing subdivision and confirmed that the medial amygdaloid nucleus provides the major input to the DR. We also demonstrated that although amygdaloid input was not as extensive as inputs from other limbic structures such as the medial prefrontal cortex or the lateral habenular nucleus, it was comparable to input from the lateral septal nucleus. Based on these observations, we suggest that the medial amygdaloid nucleus provides substantial input to the DR and may contribute an emotional influence on sleep–wakefulness cycle or pain–stress modulation. Furthermore, it seems that the medial amygdaloid–DR projection might be anatomically and functionally distinct from the well-characterized central amygdaloid–periaqeductal gray (PAG) circuit which is essential for conditioned fear. © 2007 Elsevier B.V. All rights reserved. 1. Introduction The amygdaloid nuclear complex is an almond-shaped brain region lying between the external capsule and the hypothalamus. It is divided into two main nuclear masses: (1) a centromedial nuclear group and (2) a corticobasal group. It is well known that the central amygdaloid nucleus within the former group projects to a variety of brainstem targets that mediate specific signs of fear and anxiety (Davis, 1994). The central and medial nuclei within the same group receive, in return, serotonergic or noradrenergic innervation from the dorsal raphe (DR) or the locus coeruleus (LC) (Amaral and Insausti, 1992; Sadikot and Parent, 1990). The DR is reciprocally connected to several limbic structures (Koehler and Steinbusch, 1982; Peyron et al., 1998) and changes in serotonin (5-hydroxytryptamine, 5-HT) function ⁎ Corresponding author. Fax: +82 43 851 9329. E-mail address: [email protected] (H.S. Lee). 0006-8993/$ – see front matter © 2007 Elsevier B.V. All rights reserved. doi:10.1016/j.brainres.2007.01.081 BR A I N R ES E A RC H 1 1 4 3 ( 2 00 7 ) 1 1 6 –1 25 are closely associated with emotional behaviors (Blier and de Montigny, 1994; Pineyro and Blier, 1999). For example, the medial prefrontal cortex, whose dysfunction is closely associated with affective disorders such as schizophrenia and depressive illnesses, provides extensive input to the DR (Hajos et al., 1998; Lee et al., 2003). The lateral habenular nucleus as well as the lateral septal nucleus also contributes massive input to the DR (Peyron et al., 1998). Differential roles for various limbic structures including the hippocampus, the amygdala, and the DR in regulating feeding, memory retention, and anxiety have been reported (Carlini et al., 2004). Recent anatomical and electrophysiological studies reported that the stress-related neuropeptide corticotrophin-releasing factor (CRF) modulates the activity of serotonergic neurons in the rat DR and that the central amygdaloid nucleus contains numerous CRF-immunoreactive neurons (Lowry et al., 2000; Valentino et al., 2001; van Bockstaele et al., 1998). Although morphological studies have provided evidence that CRFcontaining amygdaloid neurons target LC dendrites (van Bockstaele et al., 1998), the projection from the amygdaloid complex to the DR has not been studied extensively (Peyron et al., 1998). One of the goals of the present study was to determine which nuclear group within the amygdaloid complex provides 117 the major input to the DR. Since sub-regions of the amygdaloid nuclear complex have been functionally differentiated, the projection pattern from these areas to each DR subdivision could ascribe functional roles to specified components of the amygdaloid–DR projection. A second goal was to determine if the amygdaloid nuclear complex provides as massive an input to the DR as other limbic structures such as the medial prefrontal cortex or the lateral habenular nucleus. We employed two retrograde tracers, gold-conjugated and inactivated wheatgerm agglutininhorseradish peroxidase (WGA-apo-HRP-gold, WG) and Fluorogold (FG), in order to investigate the distribution and the density of projections from the amygdaloid nuclear complex to subdivisions of the DR in the rat. 2. Results In the first series of experiments, a retrograde tracer, WGAapo-HRP-gold (WG), was injected into each DR subdivision (Fig. 1). Although injections of variable size within a specific DR subdivision have exhibited corresponding variation in the number of retrogradely labeled cells in the amygdaloid nuclear complex, only the cases with a confined injection Fig. 1 – WGA-apo-HRP-gold was injected within the midline DR (A, R119; and B, R123) including both dorsomedial (DRdm) and ventromedial (DRvm) subdivisions of the nucleus or unilaterally into the lateral wing (DRlw) subdivision of the nucleus (C, R106; D, R127). Note that the injection in A is displaced laterally. Immunostaining reveals clusters of 5-HT cells that help define the boundaries of the nucleus. Aq, cerebral aqueduct; arrowheads, midline; mlf, medial longitudinal fasciculus; Scale bar = 200 μm. 118 BR A I N R ES E A RC H 1 1 4 3 ( 2 00 7 ) 1 1 6 –12 5 within each DR subdivision (medio-lateral dimension, 200 μm) based on 5-HT immunostaining were utilized in the present analysis. Midline injection was made either unilaterally (Fig. 1A, R119) or in the median plane (Fig. 1B, R123) at rostral, intermediate, and caudal DR levels. The tracer was also injected into the lateral wing subdivision (Fig. 1C, R106; Fig. 1D, R127) at intermediate levels of the DR. Individual cases representing midline (Fig. 2A, R119) or lateral wing (Fig. 2B, R127) injections are depicted to show the distribution of retrogradely labeled cells along the rostrocaudal extent of amygdaloid nuclear complex (Fig. 2C, sections 1–4). The labeled cells at the ipsilateral amygdaloid nuclear complex were predominant, while ones at the contralateral side were meager. For midline injections, the majority of labeled cells were located in the medial portion of the medial amygdaloid nucleus (Fig. 2C, asterisks). On the other hand, for lateral wing injections, labeled neurons were observed at the region extending from the lateral portion of the medial amygdaloid nucleus to the commissural stria terminalis (Fig. 2C, open circles). Labeling at other regions of the amygdala including basomedial, basolateral, and cortical nuclei was minimal both for midline and for lateral wing injections. An example of retrogradely-labeled neurons in the amygdaloid nuclear complex following WG injection into midline (Figs. 3A–C, R119) or lateral wing DR (Figs. 3D–F, R127) is depicted in Fig. 3. As described, the majority of labeled cells were mainly located in the medial amygdaloid nucleus (Figs. 3A–C) following midline DR injection (R119) and in the region extending from the lateral portion of the medial amygdaloid nucleus to the commissural stria terminalis (Figs. 3D–F) following lateral wing injection (R127). The number of retrogradely labeled neurons within each amygdaloid nucleus was collected from 7–9 amygdaloid sections for various injection cases (Table 1). Following midline injection at the intermediate level of DR, the majority of labeled cells was observed at the medial portion of the medial amygdaloid nucleus, while only a few cells were found at other regions including central, basomedial, basolateral, and cortical nuclei (Table 1, R119, R120, R122, R123, R125, and R126). Following the tracer injection in the lateral wing subdivision, the majority of labeled neurons was observed at the lateral portion of the medial amygdaloid nucleus as well as the region surrounding the commissural stria terminalis within the central amygdaloid nucleus (Table 1, R106, R108, R109, R127, R124, and R130). Injection at caudal midline of DR produced a pattern of labeling similar to intermediate midline injection cases, although the number of labeled cells in the former cases was less than that in the latter (Table 1, R114, R111, R113, R128, R112, and R118). The total number of labeled cells following tracer injection in the rostral midline was extremely limited (Table 1, R103, R104, R105, R115, R107, and R110). In the second series of experiments, a retrograde tracer, Fluorogold, was injected into the midline DR. Iontophoretic injection of FG produced a relatively large, but confined (mediolateral dimension, 800 μm) injection site, which involved the dorsomedial and ventromedial regions of the midline DR as well as portions of lateral wing subdivision (Fig. 4A, R129). Although the tracer is taken up by axon terminals (Fig. 4A; inset, open arrows) and transported to the Fig. 2 – Following unilateral WG injection into midline (A, R119) or lateral wing (B, R127) subregions of DR, the location of retrogradely-labeled cells was plotted on a rostro-caudal series (C, sections 1–4) of ipsilateral amygdaloid sections. Each asterisk or open circle represents one retrogradely-labeled cell body. Arrowheads, external capsule; BL, basolateral nucleus; BM, basomedial nucleus; Ce, central nucleus; Co, cortical amygdaloid nucleus; cst, commissural stria terminalis; ec, external capsule; f, fornix; ic, internal capsule; LH, lateral hypothalamus; Me, medial amygdaloid nucleus; opt, optic tract; st, stria terminalis. afferent sites, the extent of the injection was delineated by the cluster of FG-immunostained somata (Fig. 4A; inset, asterisks). The retrogradely labeled neurons were observed at several BR A I N R ES E A RC H 1 1 4 3 ( 2 00 7 ) 1 1 6 –1 25 119 Fig. 3 – The majority of retrogradely-labeled cells were observed at the medial portion of the medial amygdaloid nucleus following midline DR injection (A–C, R119), whereas neurons were observed in the region extending from the lateral portion of the medial amygdaloid nucleus to the commissural stria terminalis following lateral wing injection (D–F, R127). B/C and E/F represent higher magnification views of WG-labeled neurons (curved, open arrows) in A and D, respectively. Ce, central amygdaloid nucleus; cst, commissural stria terminalis; Me, medial amygdaloid nucleus; opt, optic tract. Scale bars in A and D represent 50 μm, whereas those in B, C, E, and F signify 10 μm. limbic structures including the medial prefrontal cortex (Fig. 4B), the lateral habenular nucleus (Fig. 4C), and the lateral septal nucleus (Fig. 4D). The positive immunostaining at these afferent sites was distinct from the surrounding regions which exhibited none of the neuronal labeling (Figs. 4B–D). An example of retrogradely-labeled neurons within the amygdaloid nuclear complex following FG injection into the midline DR is depicted in Fig. 5. The majority of FG-labeled cells were observed at rostral (Figs. 5A–C, R129) and intermediate (Figs. 5D–F, R134) levels of the medial amygdaloid nucleus. Higher magnification views of multipolar neurons often exhibited distinct dendritic morphology, which extended up to 100 μm from the somata (Figs. 5C and F). The total number of retrogradely labeled neurons in the medial amygdaloid nucleus following FG injection (Table 2) was larger than that of neurons following WG injection (Table 1); probably due to the more extensive injection site observed with FG. Thus, the number of retrogradely-labeled cells within the medial amygdaloid nucleus in the former cases was compared with that of labeled neurons located at several limbic structures such as the medial prefrontal cortex, the lateral habenular nucleus, and the lateral septal nucleus (Table 2). The total number of labeled cells in the medial amygdaloid nucleus was approximately one-fourth to one- fifth of that observed in the medial prefrontal cortex or in the lateral habenular nucleus, while it was a little less, but comparable to that of neurons in the lateral septal nucleus (Table 2). 3. Discussion Based on WGA-apo-HRP-gold tracing, we demonstrated that the input to the midline subdivision of the DR originated mainly from the medial portion of the medial amygdaloid nucleus, whereas that to the lateral wings derived from the lateral portion of the medial amygdaloid nucleus as well as the region surrounding the commissural stria terminalis within the central amygdaloid nucleus (see summary diagram—Fig. 6); the labeling was predominantly ipsilateral with only a few cells at the contralateral side. Tracing with Fluorogold confirmed that relative to other subdivisions of the amygdaloid nuclear complex, the medial amygdaloid nucleus provided the major input to the DR. We also demonstrated that although amygdaloid input was not as extensive as inputs from other limbic structures such as the medial prefrontal cortex or the lateral habenular nucleus, it was comparable to input from the lateral septal nucleus. 120 BR A I N R ES E A RC H 1 1 4 3 ( 2 00 7 ) 1 1 6 –12 5 Table 1 – Retrogradely-labeled cells were observed at various regions of the amygdaloid nuclear complex following WGAapo-HRP-gold injection into each subdivision of the dorsal raphe nucleus DR injection sites Intermediate midline Lateral wing Caudal midline Rostral midline 3.1. Cases (sex) R119 (♂) R120 (♂) R122 (♂) R123 (♀) R125 (♀) R126 (♀) R106 (♂) R108 (♂) R109 (♂) R127 (♀) R124 (♀) R130 (♀) R114 (♂) R111 (♂) R113 (♂) R128 (♀) R112 (♀) R118 (♀) R103 (♂) R104 (♂) R105 (♂) R115 (♀) R107 (♀) R110 (♀) Distribution of labeled cells at each amygdaloid nucleus Medial Central Basomedial Basolateral Cortical 25 35 19 22 14 18 13 15 18 15 20 17 11 9 7 10 8 9 2 3 1 3 2 1 5 6 3 4 3 5 8 10 12 9 11 10 2 3 3 3 2 4 1 0 0 2 0 0 3 4 2 2 3 2 0 1 2 1 2 2 2 1 0 0 1 0 1 0 1 0 1 1 2 1 1 0 1 0 1 0 1 2 0 1 0 0 1 2 0 1 0 0 1 1 0 1 2 0 1 0 1 1 0 0 2 0 1 1 1 0 0 2 0 0 1 1 0 2 0 1 Technical considerations Compared with other tracers (such as cholera-toxin B subunit) used to investigate various inputs to the DR (Peyron et al., 1998), WG produced a smaller, well-circumscribed injection site, where diffusion of the tracer into adjacent subdivisions of the nuclei was negligible. Several precautions were taken to minimize leakage of the tracer along the injection pathway from the dural surface. First, pipette tip diameters were kept small (10–15 μm) so as to minimize unwanted release. Second, we injected tracer substances in small increments over 30 min, since retrograde labeling with WGA-HRP or WG is most successful when tracer substances are injected slowly over extended periods. There was still some concern that tracer could leak from the pipette as it passed through the superior colliculus en route to DR sub-regions, thus causing false-labeling. The WG, like WGA-HRP, does not seem to be absorbed and transported via fibers-of-passage (Basbaum and Menetrey, 1987). Thus, in cases with midline DR injections, leakage of tracer within the decussation of the superior colliculus was not a concern. For the lateral wing injection, further precautions were taken to minimize leakage of tracer along the pipette track. First, a small amount of oil was backfilled into the tip of the pipette after it had been filled with the tracer so that tissue along the pipette track would not be exposed to the tracer during the process of pipette insertion. Second, following complete delivery of the tracer substance, the pipette was lowered to a point 0.1 mm deep to the original location and a small amount of extracellular fluid was back-filled into the pipette before it was withdrawn from the brain. In practice, the major problem with lateral wing injections was not spillage along the pipette track, but rather variable diffusion of tracer into the surrounding periaqueductal gray matter (PAG). Thus, the DR injection sites were processed for 5-HT immunostaining to confirm the exact location of the tracer infusion within each DR subdivision (Fig. 1). Tracer spillage into the aqueduct or the 4th ventricle was most likely cleared via cerebrospinal fluid circulation, since black granules were not detected along the ependymal layer adjacent to the injection site. Previously published reports describe only a few retrogradely-labeled cells in the amygdala following substantial horseradish peroxidase (HRP) or wheatgerm agglutinin-horseradish peroxidase (WGA-HRP) injections in the DR (Aghajanian and Wang, 1977; Kalen et al., 1985). In these previous studies, the method of approaching the DR obliquely through the cerebellum was not optimal for involving the entire dorsoventral extent of the midline DR. Such experiments were also disadvantaged because of the longer distance from the dural surface to the DR target and possible bleeding caused by passage of the pipette through the confluence of superior sagittal and transverse sinuses. We approached the DR vertically at midbrain and pontine levels by dual ligation and midline incision of the superior sagittal sinus and thus, were able to inject the tracer within the narrow confines of each DR subdivision. Scarcity of retrogradely labeled cells within the amygdala in the previous studies might also have been caused by the instability of HRP or WGA-HRP reaction product. The tracer used in the present study is known to produce a more stable reaction product due to the inactivated enzyme, WGAapo-HRP (Basbaum and Menetrey, 1987). The present study further demonstrated that among subdivisions of the amygdaloid nuclear complex, the medial nucleus provides substantial input to the DR (Tables 1 and 2). BR A I N R ES E A RC H 1 1 4 3 ( 2 00 7 ) 1 1 6 –1 25 121 Fig. 4 – Iontophoretic injection of Fluorogold (FG) into the midline DR (A, R129) produced an injection site involving dorsomedial (DRdm) and ventromedial (DRvm) regions as well as portions of lateral wing subdivision (DRlw). Although the retrograde tracer was taken up by axon terminals (inset in A, open arrows) within the DR, the efficacy of iontophoresis is often correlated with the number of labeled somata (inset in A, asterisks) which have taken up the tracer during the process. Retrogradely-labeled neurons were observed at several limbic structures such as the medial prefrontal cortex (B), the lateral habenular nucleus (C), and the lateral septal nucleus (D). Insets in B–D represent higher magnification views of FG-labeled cells. Aq, cerebral aqueduct; Arrowheads, midline; DP, dorsal peduncular region of the medial prefrontal cortex; D3V, dorsal third ventricle; fmi, forceps minor corpus callosum; gcc, genu of corpus callosum; IL, infralimbic region; LHb, lateral habenular nucleus; LS, lateral septal nucleus; LV, lateral ventricle; MHb, medial habenular nucleus; mlf, medial longitudinal fasciculus; PV, paraventricular thalamic nucleus; sm, stria medullaris; I–VI, first to sixth cortical layers. Scale bars in A–D represent 100 μm, whereas those at insets in A–D signify 25 μm. A previous study using cholera toxin b subunit (CTb) injection, however, reported that the central nucleus of the amygdala provided the major input to ventromedial or dorsomedial parts of the central DR (Peyron et al., 1998). Such discrepancies might be explained by the fact that CTb injection in the previous study (cf. Fig. 1 in Peyron et al., 1998) was large enough to involve the surrounding PAG. Because CTb is a very sensitive tracer, any spillage of the tracer into the PAG might have caused some degree of artifact labeling at the central amygdaloid nucleus. 3.2. Functional implications of amygdaloid projections to the DR The medial amygdaloid nucleus is a sexually dimorphic area and a portion of a neural pathway that regulates reproductive behavior in animals (Rasia-Filho et al., 1999; Stark et al., 1998). It is also known that the medial amygdala is involved in agonistic behavior by affecting social learning processes (Coolen and Wood, 1998; Luiten et al., 1985). The medial amygdaloid nucleus receives input from olfactory and vomeronasal systems as well as gonadal hormone inputs (Gomez and Newman, 1992). From our material it is evident that the medial amygdaloid–DR projection is not a sexually dimorphic pathway (Tables 1 and 2). The involvement of the DR in the production of behavioral and physiological responses to pain and stress has been suggested, thus emotional influences on pain and stress might be modulated via the medial amygdaloid–DR projection system (Kirouac et al., 2004; Valentino et al., 2001). Further electrophysiological studies, however, need to be performed to determine whether medial amygdaloid–DR projection might be involved in a certain type of mating behavior. 122 BR A I N R ES E A RC H 1 1 4 3 ( 2 00 7 ) 1 1 6 –12 5 Fig. 5 – Labeled cells were observed mainly at the rostral (A–C, R129) and intermediate (D–F, R134) levels of the medial amygdaloid nucleus (Me) following injection of FG into the midline DR. B/C and E/F represent higher magnification views of FG-labeled neurons (arrows) in A and D, respectively. LH, lateral hypothalamus; Me, medial amygdaloid nucleus; opt, optic tract. Scale bars in A, B, D, and E represent 100 μm, whereas those in C and F signify 10 μm. Previous reports indicated that cells in the medial amygdaloid nucleus are immunoreactive for tyrosine hydroxylase as well as vasopressin (Asmus and Newman, 1993; Caffe and van Leeuwen, 1983). Morphologically distinct populations of neuropeptide Y-immunoreactive neurons are distributed in the medial amygdaloid nucleus (Gustafson et al., 1986). A large number of NADPH-diaphorase positive neurons that might Table 2 – Retrogradely labeled cells were observed at various limbic structures following Fluorogold injection into the midline DR Cases (sex) Limbic structures Medial Medial Lateral Lateral amygdaloid prefrontal habenular septal nucleus cortex nucleus nucleus R129 R174 R179 R134 R181 R182 (♂) (♂) (♂) (♀) (♀) (♀) 47 61 55 52 45 34 196 251 238 243 189 147 204 287 251 224 199 167 65 78 63 67 56 46 exert an influence on the neuroendocrine secretion system were also found in the medial amygdaloid nucleus (Tanaka et al., 1997). Establishing the neurochemical identity of medial amygdaloid–DR projection neurons will be an important step in further characterizing the functional significance of this system. Experiments using Fluorogold confirmed that the medial amygdaloid nucleus provides the major input to the DR (Fig. 5). We also demonstrated that although amygdaloid input to DR was not as extensive as inputs from other limbic structures such as the medial prefrontal cortex or the lateral habenular nucleus, it was comparable to input from lateral septal nucleus (Table 2). Functional significance associated with predominance of medial prefronto-cortical or lateral habenular input to the DR over amygdaloid or lateral septal one could not be assessed in the present study. Another series of experiments, however, were performed in the present study to investigate the possibility that portions of the medial amygdaloid–DR projection might be influenced by the medial prefronto-cortical input to the amygdala. An anterograde tracer, PHA-L, was injected into the prefrontal cortex and WG was subsequently injected into the midline DR; BR A I N R ES E A RC H 1 1 4 3 ( 2 00 7 ) 1 1 6 –1 25 Fig. 6 – Schematic diagram illustrates a crude topographic projection from the amygdaloid nuclear complex to midline or lateral wing (lw) subdivisions of the DR. BL, basolateral nucleus; BM, basomedial nucleus; Ce, central nucleus; Co, cortical amygdaloid nucleus; cst, commissural stria terminalis; ec, external capsule; LH, lateral hypothalamus; Me, medial amygdaloid nucleus; opt, optic tract. PHA-L-labeled axonal varicosities originating from the prefrontal cortex were sparse in the medial amygdaloid nucleus, where WG-labeled, DR-projecting cells were observed (unreported observation). Thus, it would appear that the medial amygdaloid–DR pathway is not heavily influenced by the medial prefrontal cortex, despite the fact that the medial prefrontal cortex is a major source of afferent inputs to DR (Lee et al., 2003). Since the entorhinal cortex has significant projections to the medial amygdala (McDonald and Mascagni, 1997), the influence of entorhinocortical–amygdaloid projection over the DR system needs to be further evaluated in future studies. Based on highly restricted injection of the WG tracer within the lateral wing subdivision of DR, we demonstrated that amygdaloid neurons projecting to the lateral wing DR are located within the region extending from the lateral portion of the medial amygdaloid nucleus into the commissural stria terminalis (Fig. 2C, sections 2 and 3). The retrograde labeling within the major, medial and lateral portions of the central amygdaloid nucleus, however, was extremely limited. Therefore, the amygdaloid–DR projection seems to be antomically and functionally distinct from the extensive, central amygdaloid–PAG projection which is involved in conditioned fear (Davis, 1994). Previous immunocytochemical studies demonstrated that approximately half of the neurons in the medial division of the central amygdaloid nucleus contained GABA (Jongen-Relo and Amaral, 1998). CRF-like immunoreactivity has also been observed in the medial and lateral portions of the central amygdaloid nucleus (Sakanaka et al., 1986; van Bockstaele et al., 1998). Neurochemical identification of cells in the CST region projecting to the lateral wing of the DR will provide further insight regarding the functional role of this projection. 123 The present study also demonstrated that the medial amygdaloid nucleus provides less extensive input to the caudal midline than to the intermediate midline DR (Table 1). Although the direct projection from the amygdaloid nuclear complex to the caudal DR is not substantial, there is still a possibility that the caudal midline DR is influenced by the amygdala via intranuclear interconnections from lateral wing or intermediate midline DR to caudal DR (Fite and Janusonis, 2001; Fite et al., 1999; Janusonis and Fite, 2001). The amygdaloid nuclear complex provides only a few projections to the rostral midline DR (Table 1). The rostral pole of the DR at the level of the oculomotor or the trochlear nuclei is a unique subdivision in that it exhibits the smallest diurnal variation of c-Fos expression in the Mongolian gerbil (Janusonis and Fite, 2001). The rostral midline subdivision sends projections mainly to the caudate–putamen and has a reciprocal connection with the dorsomedial part of the substantia nigra, suggesting an integrative role for this subdivision in motor control (Imai et al., 1986; Pasquier et al., 1977). Based on these observations, we conclude that relative to other subdivisions of the amygdaloid nuclear complex, the medial amygdaloid nucleus provides substantial input to the DR and may contribute an emotional influence on sleep– wakefulness cycle and/or pain–stress modulation. Furthermore, it seems that the medial amygdaloid–DR projection might be anatomically and functionally distinct from the wellcharacterized central amygdaloid–PAG circuit which is essential for conditioned fear. 4. Experimental procedures A total of 34 (male and female in equal numbers for injections at each DR subdivision) Sprague–Dawley rats whose weight ranged from 300 to 350 g were used in this study. Twenty-four animals were used for WGA-apo-HRP-gold injections, while six were for Fluorogold studies. Additional four animals were utilized for prefrontocortico-amygdalo-DR projection as described in the Discussion section. Prior to surgery, each rat was anesthetized with an intraperitoneal injection of chloral hydrate (3.6% in distilled water, 1 ml/100 g body weight). All animals used in this study were treated according to guidelines approved by the institutional animal care and use committee and conformed to the NIH guidelines on care and use of animals in research. 4.1. DR injection site After anesthesia, rats were placed in a stereotaxic frame with the dorsal surface of the skull adjusted in the horizontal plane. The skull around bony lambda was removed and the superior sagittal sinus ligated with surgical sutures rostrally and caudally. Angiovasectomy was performed between suture points in order to expose the cerebral fissure at midline. The retrograde tracer, WGA-apo-HRP-gold, was injected into midline (n = 6) and lateral wing (n = 6) at intermediate DR level as well as rostral (n = 6) and caudal (n = 6) midline DR, according to the atlas of Paxinos and Watson (1998). At the rostrocaudal 124 BR A I N R ES E A RC H 1 1 4 3 ( 2 00 7 ) 1 1 6 –12 5 dimension, the DR existed approximately between 0.5 mm rostral and 1.5 mm caudal to the bony lambda. Thus injection into midline at intermediate DR level was often targeted at 0.5 mm caudal to the bony lambda at the depth of 5.6–5.7 mm, whereas the lateral wing was at 0.3 mm lateral from the midline at the depth of 5.3–5.5 mm at the same rostro-caudal dimension. The rostral midline DR was approached at 0.3– 0.1 mm rostral to the bony lambda at the depth of 5.3–5.5 mm, whereas the caudal DR was at 1.1–1.3 mm caudal to the lambda at the depth of 5.8–6.0 mm. 4.2. WGA-apo-HRP-gold injection The WGA-apo-HRP-gold was synthesized using inactivated wheatgerm agglutinin-horseradish peroxidase (WGA-HRP; Sigma, L-0390) and 20 nm (Sigma, G1652) colloidal gold (Basbaum and Menetrey, 1987). The injection apparatus consisted of a glass micropipette (tip diameter, 15–20 μm) hydraulically linked to a 2.0 μl Hamilton syringe. A total volume of 0.2 μl of WGA-apo-HRP-gold was pressure-injected into a single site within each DR subdivision over a 30-min period. 4.3. Fluorogold injection A solution of 1% Fluorogold (Fluorochrome Inc.) was prepared in saline, drawn into the tip of a glass micropipette (tip diameter, 10–15 μm) via capillary action and deposited within midline (n = 6) at intermediate DR level using a 2 μA alternating current applied on a 5-s duty cycle for 20 min through a silver lead wire (Stoelting, 50880) inserted in the pipette. 4.4. Perfusion-fixation and silver enhancement reaction After a survival period of 48–72 h following WGA-apo-HRPgold or FG injections, the animals were perfused using 150 ml of saline followed by 600 ml of fixative containing 4% paraformaldehyde in 0.01 M phosphate-buffered saline (PBS, pH 7.4). The perfusion-fixation was completed with 100 ml of PBS containing 10% sucrose. The brain was then removed and stored in 30% sucrose solution in PBS overnight. A series of 40 μm sections were made using a cryostat and every 5th section was collected in a tissue-culture plate. Following rinses with distilled water, the tracer was detected using a commercial silver intensification kit (Sigma, SE-100) as described in Llewellyn-Smith et al. (1992). 4.5. Fluorogold immunocytochemistry Sections were washed with 0.02 M potassium phosphatebuffered saline (KPBS, pH 7.4) and incubated in 1:500 dilution of rabbit anti-Fluorogold (Fluorochrome Inc.) dissolved in KPBS, which contains 1% normal goat serum, 0.3% Triton, and 1% bovine serum albumin (BSA) for 48 h (4 °C). Following rinses with KPBS, sections were then incubated in 1:250 dilution of biotinylated goat anti-rabbit antiserum (Vector, BA-1000) made with KPBS for an hour. After rinses, sections were incubated in ABC complex for an hour and then reacted with 3, 3′-diaminobenzidine (DAB)-H2O2 kit (Vector, SK-4100) for 1–2 min (at 4 °C). Acknowledgment This work was supported by an intramural grant from Konkuk University to HSL in 2005. REFERENCES Aghajanian, G.K., Wang, R.Y., 1977. Habenular and other midbrain raphe afferents demonstrated by a modified retrograde tracing technique. Brain Res. 122, 229–242. Amaral, D.G., Insausti, R., 1992. Retrograde transport of 3 D-[ H]-aspartate injected into the monkey amygdaloid complex. Exp. Brain Res. 88, 375–388. Asmus, S.E., Newman, S.W., 1993. Tyrosine-hydroxylase mRNA-containing neurons in the medial amygdaloid nucleus and the reticular nucleus of the thalamus in the Syrian hamster. Brain Res. Mol. Brain Res. 20, 267–273. Basbaum, A.I., Menetrey, D., 1987. WGA-apo-HRP-gold: A new retrograde tracer for light- and electron-microscopic single- and double-label studies. J. Comp. Neurol. 261, 306–318. Blier, P., de Montigny, C., 1994. Current advances and trends in the treatment of depression. Trends Pharmacol. Sci. 15, 220–226. Caffe, A.R., van Leeuwen, F.W., 1983. Vasopressinimmunoreactive cells in the dorsomedial hypothalamic region, medial amygdaloid nucleus and locus coeruleus of the rat. Cell Tissue Res. 233, 23–33. Carlini, V.P., Varas, M.M., Cragnolini, A.B., Schioth, H.B., Scimonelli, T.N., de Barioglio, S.R., 2004. Differential role of the hippocampus, amygdala, and dorsal raphe nucleus in regulating feeding, memory, and anxiety-like behavioral responses to ghrelin. Biochem. Biophys. Res. Commun. 313, 635–641. Coolen, L.M., Wood, R.I., 1998. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. J. Comp. Neurol. 399, 189–209. Davis, M., 1994. The role of the amygdala in emotional learning. Int. Rev. Neurobiol. 36, 225–266. Fite, K.V., Janusonis, S., 2001. Retinal projection to the dorsal raphe nucleus in the Chilean degus (Octodon degus). Brain Res. 895, 139–145. Fite, K.V., Janusonis, S., Foote, W., Bengston, L., 1999. Retinal afferents to the dorsal raphe nucleus in rats and Mongolian gerbils. J. Comp. Neurol. 414, 469–484. Gomez, D.M., Newman, S.W., 1992. Differential projections of the anterior and posterior regions of the medial amygdaloid nucleus in the Syrian hamster. J. Comp. Neurol. 317, 195–218. Gustafson, E.L., Card, J.P., Moore, R.Y., 1986. Neuropeptide Y localization in the rat amygdaloid complex. J. Comp. Neurol. 251, 349–362. Hajos, M., Richards, C.D., Szekely, A.D., Sharp, T., 1998. An electrophysiological and neuroanatomical study of the medial prefrontal cortical projection to the midbrain raphe nuclei in the rat. Neuroscience 87, 95–108. Imai, H., Steindler, D.A., Kitai, S.T., 1986. The organization of divergent axonal projections from the midbrain raphe nuclei in the rat. J. Comp. Neurol. 243, 363–380. Janusonis, S., Fite, K.V., 2001. Diurnal variation of c-Fos expression in subdivisions of the dorsal raphe nucleus of the Mongolian gerbil (Meriones unguiculatus). J. Comp. Neurol. 440, 31–42. Jongen-Relo, A.L., Amaral, D.G., 1998. Evidence for a GABAergic BR A I N R ES E A RC H 1 1 4 3 ( 2 00 7 ) 1 1 6 –1 25 projection from the central nucleus of the amygdala to the brainstem of the macaque monkey: a combined retrograde tracing and in situ hybridization study. Eur. J. Neurosci. 10, 2924–2933. Kalen, P., Karlson, M., Wiklund, L., 1985. Possible excitatory amino acid afferents to nucleus raphe dorsalis of the rat investigated with retrograde wheat germ agglutinin and D-[3H]-aspartate tracing. Brain Res. 360, 285–297. Kirouac, G.J., Li, S., Mabrouk, G., 2004. GABAergic projection from the ventral tegmental area and substantia nigra to the periaqueductal gray region and the dorsal raphe nucleus. J. Comp. Neurol. 469, 170–184. Koehler, C., Steinbusch, H., 1982. Identification of serotonin and non-serotonin containing neurons of the midbrain raphe projecting to the entorhinal area and the hippocampal formation. A combined immunohistochemical and fluorescent retrograde tracing in the rat brain. Neuroscience 7, 951–975. Lee, H.S., Kim, M.A., Valentino, R.J., Waterhouse, B.D., 2003. Glutamatergic afferent projections to the dorsal raphe nucleus of the rat. Brain Res. 963, 57–71. Llewellyn-Smith, I.J., Pilowsky, P., Minson, J.B., 1992. Retrograde tracers for light and electron microscopy. In: Bolam, J.P. (Ed.), Experimental Neuroanatomy. Oxford Univ. Press, Oxford, pp. 31–59. Lowry, C.A., Rodda, J.E., Lightman, S.L., Ingram, C.D., 2000. Corticotropin-releasing factor increases in vitro firing rates of serotonergic neurons in the rat dorsal raphe nucleus: evidence for activation of a topographically organized mesolimbocortical serotonergic system. J. Neurosci. 20, 7728–7736. Luiten, P.G., Koolhaas, J.M., de Boer, S., Koopmans, S.J., 1985. The cortico-medial amygdale in the central nervous system organization of agonistic behavior. Brain Res. 332, 283–297. McDonald, A.J., Mascagni, F., 1997. Projections of the lateral entorhinal cortex to the amygdale: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 77, 445–459. Pasquier, D.A., Kemper, T.L., Forbes, W.B., Morgane, P.J., 1977. 125 Dorsal raphe, substantia nigra and locus coeruleus: interconnections with each other and the neostriatum. Brain Res. Bull. 2, 323–339. Paxinos, G., Watson, C., 1998. The Rat Brain in Stereotaxic Coordinates. Academic Press, New York. Peyron, C., Petit, J.M., Rampon, C., Jouvet, M., Luppi, P.H., 1998. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience 82, 443–468. Pineyro, G., Blier, P., 1999. Autoregulation of serotonin neurons: Role in antidepressant drug action. Pharm. Rev. 51, 533–591. Rasia-Filho, A.A., Londero, R.G., Achaval, M., 1999. Effects of gonadal hormones on the morphology of neurons from the medial amygdaloid nucleus of rats. Brain Res. Bull. 48, 173–183. Sadikot, A., Parent, A., 1990. The monoaminergic innervation of the amygdala in the squirrel monkey: an immunohistochemical study. Neuroscience 36, 431–447. Sakanaka, M., Shibasaki, T., Lederis, K., 1986. Distribution and efferent projections of corticotrophin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 382, 213–238. Stark, C.P., Alpern, H.P., Fuhrer, J., Trowbridge, M.G., Wimbish, H., Smock, T., 1998. The medial amygdaloid nucleus modifies social behavior in male rats. Physiol. Behav. 63, 253–259. Tanaka, M., Ikeda, T., Hayashi, S., Iijima, N., Amaya, F., Ibata, Y., 1997. Nitrergic neurons in the medial amygdale project to the hypothalamic paraventricular nucleus of the rat. Brain Res. 777, 13–21. Valentino, R.J., Liouterman, L., Van Bockstaele, E.J., 2001. Evidence for regional heterogeneity in corticotrophin-releasing factor interactions in the dorsal raphe nucleus. J. Comp. Neurol. 435, 450–463. van Bockstaele, E.J., Colago, E.E.O., Valentino, R.J., 1998. Amygdaloid corticotrophin-releasing factor targets locus coeruleus dendrites: Substrate for the co-ordination of emotional and cognitive limbs of the stress response. J. Neuroendocrinol. 10, 743–757.