* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Functional Organization in the Motor Cortex
Affective neuroscience wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Haemodynamic response wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Neuroanatomy wikipedia , lookup
Aging brain wikipedia , lookup
Neural oscillation wikipedia , lookup
Cortical cooling wikipedia , lookup
Mirror neuron wikipedia , lookup
Human brain wikipedia , lookup
Brain–computer interface wikipedia , lookup
Environmental enrichment wikipedia , lookup
Development of the nervous system wikipedia , lookup
Central pattern generator wikipedia , lookup
Neurophilosophy wikipedia , lookup
Neural coding wikipedia , lookup
Response priming wikipedia , lookup
Nervous system network models wikipedia , lookup
Neuroplasticity wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Time perception wikipedia , lookup
Neuroeconomics wikipedia , lookup
Synaptic gating wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Optogenetics wikipedia , lookup
Neuroesthetics wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
C1 and P1 (neuroscience) wikipedia , lookup
Functional magnetic resonance imaging wikipedia , lookup
Metastability in the brain wikipedia , lookup
Inferior temporal gyrus wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Motor cortex wikipedia , lookup
Functional Organization in the Motor Cortex
Thesis submitted for the degree of
“Doctor of Philosophy”
By
Michal Eisenberg
Submitted to the Senate of the Hebrew University of Jerusalem
April 2012
This work was carried out under the supervision of:
Prof. Ehud Zohary
&
Prof. Eilon Vaadia
Acknowledgments
First of all I would like to thank my advisors, Eilon - for introducing me to the world of
research in neuroscience, and Udi - thanks for taking a chance, both on this project and
on me personally. Thanks for the guidance and for the encouragement when I was having
doubts.
Tanya – thanks for your patients and for sharing your tremendous knowledge. Thanks for
losing sleep over every question I had as if it were your own.
Ayelet and Tal – thanks for the much needed graphic consultation.
Itai – thank you for letting me use your data, and for investing time to help me whenever
I needed.
I especially would like to thank Lior for the wonderful collaboration. I learned a lot from
you. It was a pleasure working with you, and I hope we get to work together again.
Thank to everybody in the lab for listening, giving advice, helping me run experiments
and volunteering as subjects. Tamar, Alit, Ayelet, Lior, Yoni, Tal, Yuval, Elior, Tanya
and Zvi – thanks for making this time memorable.
To my husband Ohad – thanks for coming with me to the scanner on weekends at a
moment’s notice. Thanks for being there for me and sharing moments of despair as well
as moments of joy. Thank you so much.
Last, to my parents who have been dying to see me finish, almost as much as I have –
thanks for everything.
Abstract
The representation of reaching movements in the macaque brain has been studied extensively for
several decades using electrophysiological methods. Primary motor cortex (M1) neurons have
been shown to encode many parameters of arm movements, including movement direction.
Lately, the representation of reaching movements has been studied in the human brain as well
using
different
non-invasive
methods,
including
electroencephalography
(EEG),
magnetoencephalography (MEG) and functional magnetic resonance imaging (fMRI). I continue
this trend here by studying directionality of reaching movements in humans using fMRI, and
attempt to bridge over the gap between electrophysiological data from monkeys and imaging
data from humans.
Hubel and Wiesel have showed in the 1960’s that neurons in the primary visual cortex are
organized according to their orientation preference; i.e., neurons that respond most strongly to a
certain orientation are most likely to be clustered with neighboring neurons that have a similar
preferred orientation. Since then, it has been shown that neurons in the medial temporal cortex
(MT) are organized according to their preference for observed motion direction, and primary
auditory cortex is organized according to frequency preference. In M1, functional organization
has been studied much less extensively, but there is some evidence towards a certain degree of
organization according to neuronal preference towards the direction of hand movement in
monkeys. In humans, functional organization has yet to be studied.
To that end, a fast event related fMRI paradigm was conducted in which participants used a
joystick to move a cursor from a central origin to one of five equidistant targets. The goal was to
find directional preference in voxels, at a scale of several millimeters. Since each voxel is
thought to encompass about a million neurons, if these neurons are distributed uniformly within
the voxel, we would expect a flat tuning curve; i.e., the activation of that voxel would be the
same for all directions. On the other hand, a directional preference at such a coarse resolution
suggests that the neurons within the voxel are clustered according to their preferred direction
(PD). The rationale is that clustering would decrease the number of functional units within a
voxel, thus allowing some bias towards one direction over others. This bias could lead to gradual
I
tuning within a voxel. My findings show that voxels in M1 are directionally tuned, suggesting
functional organization. This directional tuning was shown using several analytical tools: (1) I
showed directly that when aligning the tuning curve of voxels to their PD (defined as the
direction in which activation was highest) there was a gradual decrease in activation, as direction
was farther away from that PD. (2) I used multi-voxel pattern analysis to show that spatial
patterns of activation across voxels are highly correlated with patterns during trials in the same
direction. Moreover, the correlation between two spatial patterns decreases as the distance
between the directions increases. (3) I showed that for consecutive trials in the same direction
there is suppression in activation level during repeated trials. This effect did not decrease
gradually with directional difference between the two consecutive trials, but it suggests that the
BOLD signal is in fact sensitive to movement direction. In addition, a model was constructed to
estimate cluster size. This model estimated that cluster diameter is several hundreds of microns,
which is comparable to the cluster size estimated in other studies in monkey M1.
Given these results, the question of distinguishing between the hand movement component and
the visual components of the task remains open. Is the representation of direction actually a
representation of hand-movement direction or is there a representation of target location, or of
the cursor trajectory? To that end, I ran another experiment with two runs. The first run was
standard, as in the first study I described. In the second run, however, the cursor was rotated by
45 degrees with respect to the joystick movements. This allowed dissociation between the visual
and movement components of the task, because when aligning the target location in the standard
run with the target location in the rotation run, hand movements differ by 45 degrees, and when
the two runs are aligned to movement direction, the target location differs by 45 degrees. The
same correlation analysis was used as in the previous study, and the correlations between
visually aligned directions and motor aligned directions could now be separated. Both motor
correlations and visual correlations in M1 were significantly higher than their controls. This led
to another experiment in which participants were required to merely observe the cursor moving
towards the different targets. The visual elements of the task were the same as before, but the
participants were requested not to move their hands during the experiment, and did not have
control of the cursor. This experiment was designed to see whether the visual elements of the
task were sufficient to cause activation in M1, and moreover, cause the high correlations between
same directions. None of the participants in this case show any M1 activation, nor are there any
II
positive correlations for same direction trials. Thus, the visual elements of the task are
represented in M1 only when they carry information that is relevant to movement, such as a cue,
or online feedback.
Another open question from the first study is regarding the repetition suppression (RS) I found
for movement direction. RS is suggested to reflect the tuning properties of single neurons.
Although RS has been shown in neurons in the visual cortex in response to visual stimuli, no
such effect has been shown in motor areas. In the next study, electrophysiological data recorded
from a macaque was analyzed to find a difference between repeated and non-repeated trials in
neuronal firing rate, or in the directional tuning curve at the population level. In addition, since
the local field potential (LFP) in visual areas has also been shown to manifest RS and the LFP
signal is known as highly correlated with the BOLD signal, we would expect to find some
difference between repeated and non-repeated trials. The results show no difference whatsoever
between repeated or non-repeated trials either in single units or in LFP. No difference is found
even when we limit the dataset to directionally tuned neurons, at their PD or at their least
preferred direction (which would imply a change in tuning width). Further research is required to
fully answer the question of RS, but the lack of any neuronal repetition effect, could suggest that
the RS found in the fMRI signal of the motor cortex does not reflect neuronal responses.
III
Table of Contents
INTRODUCTION……………………………………………………………………
3
Overview of the Motor System………………………………………………
A Topographic Map of the Body in M1……………………………………..
The Role of the Arm Area of M1…………………………………………….
Columnar Organization in Monkey M1……………………………………..
FMRI and neural coding……………………………………………………..
Multi-Voxel Pattern Analysis (MVPA)……………………………...
FMRI Repetition Suppression (RS)………………………………….
Research Goals……………………………………………………………….
Functional Organization in Human M1………………………………
Visual and Motor Representation in Human M1…………………….
RS in M1…………………………………………………………......
3
4
6
7
9
11
13
14
14
15
15
METHODS…………………………………………………………………………..
17
Rapid Event-Related FMRI and Deconvolution……………………………..
Event-Related FMRI Vs Block Design………………………………
Rapid Vs Slow Event-Related Design……………………………….
Deconvolution Vs GLM……………………………………………..
Experimental Paradigm………………………………………………………
Data Analysis………………………………………………………………...
Coefficient of Variation Analysis……………………………………
Analysis of Spatial Patterns of fMRI Response…………………….
Movement Repetition Analysis……………………………………...
Estimating the Size of a Direction-Selective Cluster………………………...
Rotation – Experimental Paradigm and Data Analysis………………………
17
17
17
18
18
20
20
21
22
22
23
RESULTS…………………………………………………………………………….
26
Functional Organization of Human Motor Cortex: Directional
Selectivity for Movement…………………………………………….
The Representation of Visual and Motor Aspects of Reaching
Movements in the Human Motor Cortex…………………………….
Repetition Suppression in the Primary Motor Cortex…………………….....
26
36
45
GENERAL DISCUSSION…………………………………………………………...
63
Summary of Results………………………………………………………….
Functional Organization of Human Motor Cortex:
Directional Selectivity for Movement…………………….….
The Representation of Visual and Motor Aspects of
Reaching Movements in the Human Motor Cortex………….
Repetition Suppression in M1………………………………………..
63
63
63
63
Human FMRI vs. Monkey Electrophysiology……………………………….
Is RS a Bottom-Up or a Top-Down Phenomenon?.............................
RS in Human and in Monkey…………………………………….......
FMRI Techniques and Their Relation to Neuronal Activity…….......
Directional Selectivity and Functional Organization in Other Areas
of the Brain…………………………………………………………..
Functional Organization of Neurons in M1…………………………………..
Visual Representation in M1…………………………………………………
Future Directions……………………………………………………………..
64
65
66
66
REFERENCES…………………………………………………………………….....
73
APPENDIX…………………………………………………………………………..
83
Supplementary Data – Eisenberg et al., 2010…..…………………………....
83
67
68
69
70
Introduction
Overview of the motor system
The control of movement is complex and involves many areas of the brain. The main motor
areas in the cortex are the primary motor cortex (M1), premotor cortex (PM) and the
supplementary motor area (SMA).
M1 is located in front of the central sulcus and its neurons project to many areas in the brain,
including PM, SMA, somatosensory areas, the basal ganglia, the cerebellum, the brain stem and
the spinal cord. Most of the projections to the spinal cord are to interneurons, and only a small
fraction of the projections are directly to motoneurons (Bortoff and Strick, 1993). It receives
inputs mainly from PM, SMA and from the primary sensory cortex.
PM is located in the frontal lobe, just anterior to M1. It projects directly to the spinal cord,
although not as much as M1, and to the striatum, the cerebellum, M1 and the SMA. PM receives
inputs from the prefrontal cortex, which is involved in working memory, and from the posterior
parietal cortex (PPC), and is involved in movements triggered by external stimuli (Weinrich and
Wise, 1982; Godschalk et al., 1985). The PPC in itself receives many sensory inputs in different
modalities as well as motor feedback. It is involved in transforming the sensory information into
a common reference frame, and plays a role in planning movement (Andersen et al., 1987). PM
can be divided in two: ventral (PMv) and dorsal (PMd). The inputs to the PMd and PMv are via
separate paths. PMd inputs go through the medial intraparietal cortex (MIP) and it is involved in
reaching (Crammond and Kalaska, 1996; Johnson et al., 1996), mainly in processes related to
movement preparation and movement selection (Weinrich and Wise, 1982; Weinrich et al.,
1984; Wise and Mauritz, 1985; Kurata and Tanji, 1986; Mauritz and Wise, 1986; Kurata and
Wise, 1988; Passingham, 1988; Riehle and Requin, 1989; Mushiake et al., 1991; Boussaoud and
Wise, 1993a; Boussaoud and Wise, 1993b; di Pellegrino and Wise, 1993; Crammond and
Kalaska, 1994; Kurata and Hoffman, 1994), while PMv inputs go through the anterior
intraparietal cortex (AIP) and it is involved mainly in grasping objects (Jeannerod et al., 1995;
3
Raos et al., 2006), though in contrary to these classic roles, it has been shown that PMd is also
involved in grasping (Raos et al., 2006).
PMv is divided into a caudal area (F4) and a rostral area (F5). F5 is involved more in grasping,
(distal arm control) (Rizzolatti et al., 1988), while F4 is thought to be involved in proximal arm
control (Fogassi et al., 1996). F5 neurons also respond to the visual perception of an action made
by someone else. Often, only observed movements identical to those controlled by a given
neuron are able to activate it ("mirror neurons") (Pellegrino et al., 1992; Gallese et al., 1996).
The SMA is located mostly in the medial surface of the hemisphere and is anterior to the leg area
of M1. SMA proper, the caudal part of the SMA, projects mainly to M1 and directly to the spinal
cord (He et al., 1993; He et al., 1995), and receives inputs from pre-SMA (the rostral part of the
traditionally defined SMA) and from the contralateral SMA. It is involved in internally generated
movements (Roland et al., 1980; Matsuzaka et al., 1992; Halsband et al., 1994), bimanual
movement (Brinkman, 1981; Serrien et al., 2002), and temporal sequences of movement, such as
finger tapping (Roland et al., 1980; Gaymard et al., 1990; Mushiake et al., 1990; Jenkins et al.,
1994; Gerloff et al., 1997; Shima and Tanji, 1998; Lee and Quessy, 2003).
All cortical motor areas are also connected with the cerebellum and the basal ganglia via the
thalamus. Damage to these structures provides evidence of their important role in modulation of
movement, online corrections and motor learning.
A topographic map of the body in M1
At the end of the nineteenth century Gustav Fritsch and Eduard Hitzig discovered that electrical
stimulation of the motor cortex produces movements on the contralateral side of the body
(Fritsch and Hitzig, 1870). They showed that stimulating different parts of the cortex activated
different groups of muscles. The excitable sites formed a topographical map of movements on
the surface of the brain. These results were soon replicated by Ferrier (1873). However, he used
stimuli with a longer duration, which were found to evoke coordinated, purposive movements,
rather than just twitches. In 1905 a detailed map of the human M1 was described by Campbell
(1905). These findings were expanded to several species of monkeys (Leyton and Sherrington,
4
1917). In the 1930s Penfield found a topographically organized representation of the entire body
in human M1 ("homunculus"), where the lips and tongue, thumb and hand receive a
disproportionally large representation (Penfield and Boldrey, 1937). A homologous map was
found in monkeys (Woolsey et al., 1952). The different joints of the arm are represented in
concentric rings in the arm area of M1, such that proximal joints are surrounding distal ones
(Murphy et al., 1978). Somatotopic maps in M1 were also generated using functional MRI
(fMRI) (Rao et al., 1995).
This topographic map may imply that each body part is represented in an orderly fashion and
occupies non-overlapping cortical space. Asanuma suggested that each cortical column in M1
controls a specific muscle, or a set of muscles (Asanuma and Ward, 1971; Asanuma and Rosen,
1972; Asanuma, 1975). However, even the maps of Penfield and Woolsey showed great overlap.
Lemon found that single neurons receive afferent input from different fingers (Lemon, 1981).
Spike-triggered averaging has shown that neurons activate more than one forelimb muscle (Fetz
and Cheney, 1980; Cheney and Fetz, 1985; Cheney et al., 1985; Buys et al., 1986; Lemon et al.,
1986; Fetz et al., 1989). In fact, neighboring neurons seem to activate similar muscle fields. It
was suggested that M1 neurons activate a functional set of muscles. Filling single corticospinal
neurons with horseradish peroxidase (HRP) led to terminal ramifications in more than one spinal
motor nucleus (Shinoda et al., 1981). This provided an anatomical basis for this divergence.
Later, Schieber & Hibbard (1993) found extensive overlap between fingers in the hand area,
suggesting that the control of each finger utilizes a population of neurons distributed throughout
the hand area rather than a somatotopically segregated population. Evidence of overlap in the
arm subregion was also found in neuroimaging studies in humans with positron emission
tomography (PET) (Colebatch et al., 1991; Grafton et al., 1991) and fMRI (Rao et al., 1995;
Sanes et al., 1995; Beisteiner et al., 2001; Dechent and Frahm, 2003; Kleinschmidt et al., 2006;
Meier et al., 2008). Meier (2008) also showed that the wrist and the forearm were relatively
emphasized both ventrally and dorsally to the finger representation, violating the traditional
somatotopic order. In addition, M1 arm sub-region has vast horizontal interconnectivity without
an obvious topographic plan for fingers, wrist, or other parts of the arm (Huntley and Jones,
1991). Magnetoencephalography in humans likewise has shown that the dipole sources of the
neuromagnetic fields generated during movements of different digits are not arrayed in
somatotopic order, either in a single subject or averaged across multiple subjects (Cheyne et al.,
5
1991; Salenius et al., 1997). Taken together, this body of experimental data reviles a pattern of
organization which has the richness and the flexibility to execute the complex movements we
make.
The role of the arm area of M1
M1 plays a fundamental role on the control of arm movements. What exactly that role is has
been the subject of a longstanding debate. In the late 1960’s, Evarts (1968) showed that neurons
in the arm area of M1 of the macaque co-vary with muscle force. Thach (1978) showed that
neurons in M1 code different parameters of movement: muscle force, joint torque and intended
direction. But these motor studies were always to one of two directions. To that end,
Georgopoulos and colleagues (1982) studied movements to eight different directions. They
found that neurons in the arm area of M1 encode movement direction in space, and that the
activity of these neurons can predict the actual direction of movement (Georgopoulos et al.,
1986; Georgopoulos et al., 1988). In 1990 directionally tuned neurons were shown to change
their preferred direction (PD) while arm movements were made in different parts of
extrapersonal space, although the population vector still pointed at the correct direction (Caminiti
et al., 1990). This suggests that coding is not entirely extrinsic. Similar results were also found
by Scott and Kalaska (1995, 1997), who used different arm postures, and by Sergio and Kalaska
(1997, 2003), who used an isometric force in different locations in the workspace. In 1999 Kakei
and colleagues (Kakei et al., 1999) designed an experiment in which they differentiated between
muscle activity, wrist joint direction, and direction in space. Interestingly, they found both a
group of neurons co-varying with muscle activity and a group of neurons co-varying with
movement direction in space, which did not change their preferred direction for different muscle
activity. Thus, both extrinsic and intrinsic parameters are coded in M1. If M1 encodes both
intrinsic and extrinsic parameters, perhaps the transformation between the goal and the execution
of the task is done in M1, at least to some extent. Therefore, M1 could be involved in a much
more complex process during movement than previously thought, which includes planning,
execution, and online correction.
At what point does M1 come into play and becomes involved in the planning? Is it only after we
know the required movement vector, or is the movement vector itself planned in M1 as well? To
6
answer this question we need to know which parameters of the goal are represented in M1. There
is evidence towards a representation of the visual cue in human (Saleh et al., 2010) and in nonhuman primates (Martin and Ghez, 1985; Alexander and Crutcher, 1990; Lurito et al., 1991;
Shen and Alexander, 1997). Some studies have even shown neuronal sensitivity to specific
features of the visual cue, such as its location or color (Zhang et al., 1997a; Zach et al., 2008).
Columnar organization in M1
The primary visual cortex (V1) has long been known to be organized in vertical columns
perpendicular to the cortical surface according to the preferred orientation of neurons (Hubel and
Wiesel, 1962). These columns were shown, using in-vivo optical imaging, to be arranged in
pinwheel-like patterns (Bonhoeffer and Grinvald, 1991). Since then, similar clustering according
to the functional properties of neurons has been shown in several other primary sensory areas of
the brain, such as somatosensory cortex (Powell and Mountcastle, 1959), primary auditory cortex
(A1) (Imig and Adrian, 1977) and middle temporal cortex (MT) (Albright et al., 1984). This has
been shown even more convincingly with voltage sensitive dye (Blasdel and Salama, 1986) and
with calcium imaging in V1 (Ohki et al., 2005) and later in A1 (Rothschild et al., 2010). Are
neurons in M1 also organized according to their functional properties? This has been a
longstanding question. M1 has a similar anatomical structure as these other cortical areas.
Therefore, we would expect the same general rules that govern the primary sensory areas to
govern M1 as well. But according to which parameters do we expect M1 to be organized? Earlier
work investigated columnar organization in M1 according to different muscles (Asanuma, 1975)
using intracortical microstimulation. Later work focused mostly on movement direction.
Clearly, the picture is not as clear cut in V1, but there is accumulating indirect evidence to
support some clustering according to the neurons’ PD. The firing rates of neighboring neurons in
M1 have been shown to be correlated (Murphy et al., 1985; Kwan et al., 1987; Vaadia et al.,
1995), suggesting that neighboring neurons share common inputs. Georgopoulos and colleagues
(1993) estimated the synaptic strength between pairs of neurons using an analysis based on
waiting time probability density function, and found that the synaptic strength was negatively
correlated with the difference between the neurons’ PDs. This was strengthened by the results of
7
Hatsopoulos (1998), who used cross-correlation techniques to show that fine temporal synchrony
carries information regarding movement direction and contributes to coding beyond the
information available from the changes in firing rate. In addition, pairs of neurons with a similar
directional preference were found to show higher noise correlation (Lee et al., 1998; Maynard et
al., 1999). Later, Ben-Shaul et al. (2003) found that neurons recorded from the same electrode
have more similar PDs than neurons recorded from distant electrodes. Amirikian and
Georgopoulos (2003) showed that cells with similar PDs tended to segregate into vertically
oriented minicolumns 50-100 µm wide and at least 500 µm high. Such minicolumns aggregated
across the horizontal dimension in a secondary structure of higher order. In this structure,
minicolumns with similar PDs were approximately 200 µm apart and were interleaved with
minicolumns representing nearly orthogonal PDs.
Finally, directional tuning has been shown in signals with a larger scale than single neurons, such
as multi-unit activity (MUA) (Stark et al., 2009), in which PDs were similar to those of single
units recorded from the same electrodes, and LFP (figure 1) (Mehring et al., 2003; Rickert et al.,
2005), which represents populations at the resolution of between 250 microns and several
millimeters (Mitzdorf, 1987; Victor et al., 1994; Kruse and Eckhorn, 1996; Gail et al., 2003;
Kreiman et al., 2006; Liu and Newsome, 2006; Logothetis et al., 2007; Berens et al., 2008;
Gieselmann and Thiele, 2008; Katzner et al., 2009; Rasch et al., 2009; Xing et al., 2009). These
data suggest some level of clustering of neurons in monkey M1 according to the neurons’ PD;
otherwise we would expect the tuning curves at large resolutions to be flat, with no bias towards
any one direction over others.
Despite the evidence of functional clustering, it is difficult to reconcile the results of these
studies with a strict columnar structure, since the difference between PDs of neurons recorded by
a single electrode was relatively large (Ben-Shaul et al., 2003; Stark et al., 2009), and nearby
neurons frequently encoded different kinematic parameters (Stark et al., 2009). Since M1
neuronal activity has been found to encode many different parameters, the functional architecture
could also be more complex than that found in sensory cortex (Hatsopoulos, 2010).
8
Figure 1: Directional tuning of a sample movement evoked potential (mEP)
obtained from a single electrode in M1 during execution of contralateral
movements. Trial averaged LFP activity is shown separately for each
movement direction. Time zero indicates movement onset. Note that this mEP
is strongly tuned, with the highest amplitude for movement to the left (180
degrees). Rickert et al. 2005
Many studies in humans show a body map in M1, where the size of each area is several
millimeters (Zeharia et al., 2012). However, there are practically no studies showing clustering in
M1 at a finer resolution. We will further discuss the ability to explore such small structures using
functional imaging, despite its relatively coarse resolution.
FMRI and neuronal coding
FMRI is widely used to study the brain, but what exactly is the nature of the BOLD fMRI signal,
and how exactly is it related to neuronal electrical activity? In 2000, Heeger and colleagues
showed that the BOLD signal in MT increases linearly with motion coherence, with a slope
comparable to that of the firing rate of single neurons (Heeger et al., 2000). Simultaneously
scanning anesthetized monkeys and recording electrical signals revealed that the BOLD signal is
much more correlated with the mid gamma range of the LFP signal (i.e. temporal frequencies 409
130Hz) than MUA or single unit activity (SUA) in visual cortex of monkeys (Logothetis et al.,
2001). This suggests that the BOLD signal reflects the inputs rather than spiking outputs, since
LFP is known to reflect synchronized input signals of the neural population within up to several
millimeters around the electrode tip (Mitzdorf, 1987). Rauch and colleagues (2008) were able to
dissociate between the MUA and the LFP signals by using a serotonin receptor agonist, which
reduces the MUA without affecting the LFP. Under these conditions, they found that the BOLD
signal was also not affected by the serotonin receptor agonist, and was better predicted by the
LFP signal.
Mukamel et al. (2005) recorded SUA and LFP from two neurosurgical patients and compared
them with the BOLD signal from healthy subjects during presentation of an identical movie
segment. Their findings show that the BOLD signal was highly correlated with the signal
predicted according to the SUA. Adding to these findings is the work of Shmuel et al. (2006),
who showed that the negative fMRI response was actually correlated with the decrease in firing
rate in V1. Additionally, Nir and colleagues (2007) reported data from simultaneous recordings
of SUA and fMRI. They found that the BOLD signal was correlated not only with the gamma
band LFP in the human auditory cortex, but it was also predicted by the correlation between
firing rates of neighboring well-isolated single neurons, suggesting that the BOLD signal may
reflect the overall local neuronal activity.
On the other hand, this link between brain hemodynamics and local neuronal activity was
challenged by the finding of a component of the hemodynamic signal that precedes the onset of a
periodic stimulus and that is independent of standard predictors based on LFP and MUA. This
trial-locked haemodynamic signal could be due to an arterial pumping mechanism. However, this
dissociation was only observed in the absence of visual stimulus. Stimulus-evoked hemodynamic
activity was highly predictable by LFP and MUA (Sirotin and Das, 2009).
Recently, electrocorticographic (ECoG) recordings from epileptic humans showed that beta and
mid gamma range LFP explain different components of the BOLD signal and that the LFP signal
in different cortical regions is differentially correlated with the BOLD response (Conner et al.,
2011). The best (pre-stimulus) baseline correlation of beta and gamma power with the BOLD
signal was found in the occipital and parietal lobes while the worst correlations were found in the
10
frontal lobe. Thus, caution should be taken when applying methods that were established in the
occipital and parietal lobes to frontal areas.
Multi-voxel pattern analysis (MVPA)
Traditionally, fMRI analysis methods have focused on cognitive variables and individual voxels.
Since the majority of fMRI studies focused on finding the anatomical loci of these cognitive
variables, these analysis methods were extremely productive. However, there are limits to
examining voxels in isolation. Haxby and colleagues (2001) were the first to introduce the
analysis of MVPA. In this method of analysis, spatially distributed patterns of activation are
compared across voxels. Conventional analysis methods were discarding a large amount of
information contained in these patterns. MVPA can pick up on much more subtle differences
because the spatial distribution of activation in response to two different stimuli can be different
even when the average response of the ROI is the same. In Haxby’s study, they compared spatial
patterns of visual stimuli between categories and within categories (e.g., faces, houses, chairs,
etc.), and found areas where correlation coefficients between pairs of stimuli from the same
category were higher than between stimuli from different categories, thereby discriminating
between the different perceptual states.
This principal was later utilized to decode grating orientation from the BOLD pattern of
activation in early visual areas in single trials (Haynes and Rees, 2005; Kamitani and Tong,
2005). It has been suggested that the reproducible yet specific patterns to the different
orientations could reflect sub-voxel tuning. The patterns may arise from variability in the
distribution of cortical feature columns, or their vascular supply (see figure 1; Boynton, 2005).
The weak biases in the voxels' fMRI responses can be used to distinguish between different
stimuli, or different types of movement.
Op de Beeck (2010) disputed this point, showing that these patterns did not diminish with
smoothing. He claimed that if the patterns were created by the distribution of orientation
columns, the decoding ability should have decreased with smoothing, as there are many more
columns in a larger, smoothed, unit. Kamitani and Sawahata (2010) responded that spatial
smoothing does not prove that the source of MVPA isn’t at the sub-voxel level by showing
11
analytically that no information is lost by spatial smoothing. Swisher and colleagues (2010)
scanned a cat using high-field high-resolution fMRI to show that the majority of the information
in multi-voxel patterns about orientation is at a spatial scale of up to a millimeter, which is also
the scale of the diameter of a column. This confirms the hypothesis that the origin of multi-voxel
patterns is the distribution of orientation columns, and not just large-scale biases. These results
were replicated by Freeman et al (2011). However, Freeman was also able to achieve similar
results for angular position (retinotopic location), a parameter which is known to be mapped at a
large scale rather than fine-scale columnar architecture. This demonstrates that spatial filtering
does not distinguish well between maps and columns. Moreover, they show a coarse-level
topographic map of orientations, which was both sufficient and necessary for decoding.
The latest paper on the subject examined the scale of organization for object selectivity in the
ventral visual cortex, both by investigating the effect of spatial smoothing on MVPA reliability
and by comparing the relative weight of higher and lower spatial frequencies. The differential
activation for category comparisons was found to be organized at a larger spatial scale than for
the within-category comparison. This finding confirms the existence of multiple scales of
organization in the ventral visual cortex (Brants et al., 2011).
An intriguing possibility was suggested by Gardner (2010): It is possible that the vasculature
system is organized around columnar architecture in a way that could amplify weak signals and
make them more easily measurable by fMRI. Cortical columns that share the same tuning
properties may often be active together and thus require oxygen and metabolic nutrients together.
A single artery could provide oxygenated blood to a number of columns. Thus, the ability of
classifiers to work at low spatial resolutions may be the consequence of a well-structured
vasculature aligned to the functional architecture of the cortex.
12
Figure 2: Patterns of orientation-selective responses measured with fMRI. The colored arrows
represent the preferred directions of neurons. Top right: synthetic orientation tuning data
generated by band-pass filtering random orientation values, assuming neurons are clustered
according to their preferred direction. The black squares represent 3 3 mm2 fMRI voxels.
Bottom right: histograms showing the proportion of selectivity inside each voxel to each of the
eight orientations shown below. This shows how different stimulus orientations produce slightly
different patterns of responses in V1. Top left: synthetic data, assuming neurons are uniformly
distributed. Bottom left: histograms showing flat tuning curves at the voxel level. Adapted from
Boynton, 2005.
FMRI repetition suppression (RS)
Repetition of the same stimulus has been shown to cause a decrease in activation compared to a
novel stimulus (e.g., Buckner et al., 1998). RS, or adaptation, was first introduced as a method
for providing information at a sub-voxel resolution by Grill-Spector and Malach (2001). The
rationale behind this technique is that a voxel’s activation is the average responses of about a
million neurons, while RS enables tagging specific neuronal populations with a certain property.
Thus, we can differentiate between a situation in which a neuronal population has the property,
and where there are several populations with different properties that are all activated a little to a
certain stimulus. An example is given by Malach (2012): if an imaged voxel contains a
13
heterogeneous and balanced mix of highly selective neurons, for example neurons narrowly
tuned to image size, the BOLD response, which pools all these selective responses together, will
consequently appear to be “size invariant”. The same will be true, of course, if the neurons in the
voxel are individually size invariant. Thus, it is impossible to decide, by observing the BOLD
response alone, what are the functional properties of the neurons. Repeated presentations of an
identical image in the same size may cause signal reduction in both cases. However, presenting a
sequence of varied sizes of the image will cause signal reduction only in the voxel containing
invariant neurons, since they are "blind" to the size change, while for the size selective neurons
each size change appears as a novel stimulus.
A decrease in activation has been shown in single neurons (Movshon and Lennie, 1979), but the
relationship between the neuronal signal and the RS phenomenon is not fully understood. Grill
Spector and colleagues (2006) proposed three possible models to explain the RS: (1) “fatigue”,
according to which all neurons show a proportionally equivalent decrease in activation in
response to a repeated stimulus. The neural mechanism in this case could either be firing rate
adaptation or synaptic depression (short term or long term) (Miller and Desimone, 1994; GrillSpector and Malach, 2001), (2) “sharpening”, where we expect fewer responsive neurons
following stimulus repetition (Li et al., 1993; Desimone, 1996; Wiggs and Martin, 1998) and (3)
“facilitation”, which predicts that repetition facilitates faster processing of the stimulus and
therefore shorter duration of neuronal firing (Sobotka and Ringo, 1996; Henson and Rugg, 2003;
James and Gauthier, 2006).
The interpretation of fMRI RS was complicated by Sawamura and colleagues (2006). They
showed that neurons in the macaque inferior temporal cortex (IT) adapt to a stimulus that they
were responsive to. However, when two stimuli which evoke a similar response were shown one
after the other (ABAB…), there was less adaptation to the second stimulus, than when the
stimuli were identical (AAAA, or BBBB). This indicates that the level of adaptation shows
greater stimulus selectivity than the magnitude of the neuronal response. These results are
inconsistent with the fatigue model, in which case adaptation would transfer across stimuli and
be purely response dependent. Therefore, the source of adaptation is probably input specific.
This hypothesis was later strengthened by De Baene and Vogels (2010), and is consistent with
evidence from V1 (Movshon and Lennie, 1979; Müller et al., 1999), but in MT for example,
14
Kohn and Movshon (2004) found sharpening of the neurons’ tuning curves, which could imply
different mechanisms in different areas, different types of stimuli, or different time scales.
Further interpretations of RS will be discussed in chapter 3 of the results, about RS in the motor
cortex.
MVPA and RS were recently compared in the visual cortex (Sapountzis et al., 2010). The
estimates of orientation selectivity obtained with the two methods of analysis were highly
correlated across visual areas. However, the MVPA approach was more sensitive to stimulus
orientations (i.e., distinguished stimuli with a smaller separation than RS).
Research goals
Functional organization in human M1
There are many studies about the functional organization in V1 (according to stimulus
orientation), in MT (according to observed motion of the stimulus) and in A1 (according to
frequency of auditory stimuli). M1 neurons are known to be tuned to movement direction, but
there is little evidence of functional organization of these neurons, and none in human M1.
Are neurons in human M1 clustered according to their PDs, or are these neurons uniformly
distributed? If neurons with similar PDs are organized in clusters, what size are these clusters? In
the first chapter, I answer these questions in two steps. First I show, using several different
methods, that voxels, encompassing about a million neurons each, are directionally tuned. I
claim that this tuning at such a coarse resolution could not have been found had the neurons been
uniformly distributed. Next, I constructed a model to estimate cluster size based on the level of
average directional tuning in M1 voxels.
Visual and motor representations in human M1
Each trial in our reaching task began with a target appearing on the screen. Then Participants had
to wait for the go signal (change of color of the cursor at the center of the screen) and then use
the joystick to move the cursor towards the target, while gaining visual feedback of the cursor
throughout the trial. The temporal resolution of the BOLD signal is not high enough to separate
15
the different epochs. This brings up the question, whether human M1 holds information only
about movement direction, or is there also a representation of a higher level of the task, such as
the visual target or online visual feedback? In the second chapter I dissociate between motor and
visual elements of the task in order to address this question by introducing a 45 degree
visuomotor rotation. My results show that the activation pattern in M1 is sensitive to the visual
components of the task, as well as to the motor components. This sensitivity to visual
components disappears in the absence of a motor task. Therefore, the visual representation in M1
is task related, and probably has to do either with the goal of the task, or with the online
feedback.
RS in M1
In the first chapter I show that the BOLD response in M1 to a repeated movement in the same
direction is reduced compared to the response during a non-repeated movement. This was also
shown in two other studies (Dinstein et al., 2007; Fabbri et al., 2010), and is added to the many
studies that show RS of the BOLD signal for repeated visual stimuli in visual areas. This raises
the question, what is the neuronal mechanism for the RS found in fMRI for movements? Is it the
same as for visual stimuli? I started by looking for either a decrease in firing rate in M1 neurons
for repeated movements, or a change in their tuning curve width (since a decrease in the BOLD
signal could reflect a decrease in either the PD and therefore, widening of the tuning curve or in
the directions near the PD and therefore, sharpening of the tuning curve). In addition, I look at
the LFP, which has been shown to be more correlated with fMRI activation, trying to find some
effect of repetition.
16
Methods
Rapid event related fMRI and deconvolution
Event-related fMRI vs. Block design
Event related fMRI is the use of fMRI to detect responses to individual trials rather than in the
more traditional block design, where the same condition is repeated for an entire block with a
much longer duration (typically ~10-30 seconds). The advantage of the event related design is
that the different conditions can be randomized and unpredictable, and the signal is less sensitive
to temporal drift (e.g. due to head motion).
Rapid vs. slow event-related design
When subjects participate in an fMRI experiment, they have to lie on their backs without moving
their heads; they are subjected to many stimuli and sometimes have to respond by pushing a
button or, as in our example, moving a joystick. In order to gain enough statistical power, there
are usually many repetitions. On top of that, because it takes the fMRI signal at least 12 seconds
to decay and go back to baseline, the original (slow-event related) fMRI paradigm required a
long waiting time until the next trial. This can be very boring. Many have reported falling asleep
at some point during the experiment. This is one of the reasons we use a rapid event-related
design, in which a new trial begins every 4 seconds (in our case). This allows many more trials in
a given session, allowing stronger statistical power. However, necessarily this causes an overlap
between the responses to subsequent trials: the BOLD response to a given trial peaks when the
participant is usually already performing the next movement. In order to analyze such data, there
are prerequisites in designing the task: 1) Eliminating the effects of recent trial history as much
as possible by using first-order counterbalancing. I.e., after each of the 5 trial conditions, there is
an even distribution of these conditions in the next trial (e.g., ~20% of trials after movement in 00
will be in the same direction, ~20% will be 450, etc.). This condition is important for ensuring
that the response to a trial type is not biased by context or by the history of preceding trial-types.
2) Null trials of various lengths are embedded in the experiment. These null trials provide jitter
17
in the inter-trial intervals, and thereby improve the statistical efficiency, i.e., the accuracy with
which the hemodynamic responses are estimated. The different sequential trials are averaged out,
and the response to a certain condition, movement direction in our case, can be distinguished
despite the overlap. This differential overlap provides a more efficient estimation than slow
event-related designs (Dale, 1999).
Deconvolution vs. GLM
To analyze the data, we use the deconvolution method. Deconvolving the overlapping
Hemodynamic Response Function (HRF) is possible according to reasonable assumptions, such
that the response to multiple stimuli is the linear sum of the response to the individual stimuli
(linear superposition assumption(Glover, 1999).
An advantage of this method is that it's model free. Generally, it's accepted that the BOLD
response to an event, usually a stimulus, is shaped as a single- or two-gamma function. The
BOLD signal we measure (i.e., the raw signal) is this HRF convolved with the sequence of
events (plus noise). We know the sequence of events and the HRF, and all that is left for us to
find is the response amplitude (β) that minimizes the noise, according to the formula: Y=bX+e,
where Y is the raw data, X is the design matrix, e is the Gaussian noise, and b is the vector of
response amplitudes to the different stimuli used in the experiment. This is the General Linear
Model (Friston et al., 1994). In the deconvolution method, instead of having to assume a
predefined shape of the BOLD response, we model the entire response function (in the temporal
domain), without any assumptions about its underlying shape. In this analysis, each condition is
represented by several β weights, 10 in our case, causing vector b to be 10 times longer. As a
result we don't have to assume that the response to movement in the motor cortex is necessarily
shaped the same as the hemodynamic response function (HRF) typically found in primary
sensory areas. It has been shown that different areas in the brain show a different response
function (Schacter et al., 1997). In addition, the BOLD response appeared to vary considerably
across different people (Aguirre et al., 1998). The deconvolution approach allows different
response functions for different participants, for different areas of the brain, and even for the
different behavioral conditions (i.e., the different movement directions). With that being said,
we still expect to see the same general shape of the HRF in the deconvolution kernel.
18
Experimental paradigm
Each trial lasted 4 s. The trial began with presentation of a red circle in the center of the screen
(“origin,” radius of 0.7°). Initially, the participants had to hold an MRI-compatible joystick still,
and make no hand movement. After an interval of 500 ms, five circles (targets, radius of 1°)
appeared at the upper half of the screen, spread around the center at equal distances, between 0°
and 180°, 45° apart. Their distance from the origin was 4.5° of visual angle. Four of the circles
were blue and one circle was green, signaling the required future direction of movement. The
participants had to keep their hand still until the “go” signal, in which the red “origin” circle
turned into a white cursor, which occurred 2 s later. They were instructed to respond by moving
the cursor toward the green target, using the joystick. Participants had 1.5 s to reach the target.
Upon reaching the target, all circles disappeared. This served as a cue for the participants to
release the joystick (thereby relaxing the spring), which resulted in the joystick returning to its
starting position at the center. There was no explicit failure signal. If the participants did not
reach the target within the 1.5 s time limit, all circles disappeared and the next trial began
(participants failed to reach the target in 10.6% of the trials).
Participants were instructed to try and make quick and accurate movements towards the targets.
They were not instructed as to how to move their arms towards the target. However, the joystick
was relatively small, and in order to make quick and accurate movements towards the target,
movements were generally limited to the wrist and hand. The verb "reach" here refers to
attaining the target by a movement, even though participants are not actually reaching with their
whole arm.
The length of the joystick is 11.5cm, and the angular range of the joystick is +/-15 degrees. Thus,
given that participants used the full extent of the joystick range, the size of the movement
required to displace the cursor from the origin to the targets was ~3 cm.
The red origin and all five blue targets appeared on the screen for 2–8 s during intermittent null
trials, in which the participants were instructed not to move the joystick until the next trial began.
19
This rapid event-related fMRI study consisted of 250 trials, 50 in each direction. The trial order
was counterbalanced (first order). Null trials were pseudorandomly embedded between
movement trials. Our constraint was that the total length of all null trials would equal the total
length of each of the five movement conditions (200 s). The trial sequences were built using
optseq software, which chooses the most efficient sequence (most variable history before each
condition) out of 10,000 random sequences sampled. The experiment began and ended with 16 s
of a null event.
The fMRI acquisition, preprocessing and defining the regions of interest will be described in the
specific methods section of the results chapters 1 and 2.
Data Analysis
Coefficient of variation analysis
We used a bootstrap analysis, to test whether the coefficient of variation (CV =SD/mean) of each
voxel's average response to the five directions of movement is significantly higher than expected
by mere chance (e.g., had there been no directional selectivity). In this analysis, we randomly
reassigned conditions (directions of movement) to the trials, while maintaining the original
proportions of hand movements (each condition was assigned to 20% of the trials). The null
conditions were not replaced. We created a new regression matrix, estimated the β values for the
different directions, and assessed the CV, separately for each assignment. This procedure was
repeated 10,000 times, resulting in a distribution of expected CVs merely due to noise. Next, for
each voxel, we assessed its p value: the fraction of CV values obtained by the bootstrap method
that were greater than the actual CV of that voxel. Finally, a χ2 test was used to show that the
distribution of p values across voxels was significantly skewed to low values (differing from a
uniform distribution, which would be expected by chance).
The CV analysis was used also for the estimation of cluster size. For this purpose, since each
voxel is expected to show some variation in its response by chance (as there were limited
repetitions of each direction of movement), we also corrected each voxel's CV by subtracting
from its actual variance in response (for the various directions) the mean variance of the
20
bootstrapped activation (XBS) across iterations:
In addition to
calculating voxel CV, we also calculated the CV as a measure of the directionality of the LFPs in
monkey M1, when monkeys performed the same center-out task [courtesy of S. Cardoso de
Oliveira (Cardoso de Oliveira et al., 2001)]. To that end, we used the peak-to-peak distance (the
distance between maximum and minimum) of the mean evoked potential, per direction of
movement. CVs of LFPs were calculated without subtracting bootstrapped variance.
Consequently, the CV is slightly overestimated.
Analysis of spatial patterns of fMRI response
To detect directional selectivity of voxel population spatial patterns, each individual's data were
split into two datasets, such that each of the 50 trials in each direction was randomly assigned to
one of the datasets. For each voxel, we estimated the β values and defined the activation for each
direction (i.e., activation value) as the average of the β values measured 6 and 8 s after the
beginning of the trial (~4–6 s after movement initiation; normally at the peak activation). Then,
in each dataset and for each voxel, we subtracted the voxel's mean activation level (across all
directions) to remove activation differences between voxels that are unrelated to movement
direction. Without such a normalization procedure, one would get high correlations between the
multivoxel spatial patterns from all comparisons, simply because some voxels are more active
than others, regardless of the direction of movement.
The above analysis resulted in two matrices, one for each dataset. Each matrix consisted of 5
columns (one for each direction) with length N equal to the number of voxels in the ROI. The
entries of each row of the matrix were the 5 activation values of a single voxel for all 5 directions
of movement.
Next, we calculated the correlation coefficient (CC) between the columns of the first and the
second datasets (each corresponding to the pattern of activation across all voxels, for a given
condition). If the activation values contain information about the direction of movement, one
should get a higher CC for movements to the same direction (in the two datasets) than the CC
calculated for movements to two different directions. To ensure that the results reflect a reliable
21
trend, and are not merely due to some arbitrary division of the trials into the two datasets, we
repeated this analysis 100 times for each participant; in each iteration, the data were split
differently into two random datasets. The results shown are the mean CC values across all 11
participants. The resulting dependence of the CC on the angular difference between the two
directions of movements matched a normal distribution:
Movement repetition analysis
The purpose of the repetition analysis was to find a decrease in activation when the same
movement is made twice in a row. This would provide additional evidence that M1 fMRI
activation is sensitive to movement direction. In this analysis, the various conditions were split
according to the angular difference between the direction of movement in the current trial and
the movement direction in the previous trial. Thus, a condition of 0° means that the movement
was to the same direction as in the previous trial. Beta values were estimated in the same way as
in the previous analyses.
To test whether a voxel's PD (direction eliciting the greatest response) is the same direction that
elicits the greatest repetition suppression (RS), we divided the trials into 10 conditions. First we
divided the trials into repeated and nonrepeated trials, and then we divided each set of trials into
five conditions according to their current direction of movement. The repetition index for each
voxel (v) and each direction (d) was defined as βv,d(nonrepeated)/βv,d(repeated).
Both repetition index and activation index (of nonrepeated trials) were normalized by subtracting
the mean and dividing by the SD across directions in each voxel (to bring the two different
signals to a common scale) and then correlated. Pairwise Student's t test was used to test whether
the CCs in the “same direction” and “different direction” conditions were significantly different.
Estimating the size of a cluster of neurons with similar PDs
Cluster size was calculated based on the estimated number of clusters in each voxel. In order to
estimate the number of clusters within each voxel, we modeled the voxels as having anywhere
22
between 5 and 1500 clusters. Each cluster was presumed to be a cluster of neurons with similar
neuronal properties, including the same PD, which was one of 8 directions, between 0 and 315
degrees, with 45 degree decrements. For each cluster, the PD was randomly chosen from a
uniform distribution of the 8 directions (see figure 1). After assigning a PD to each cluster, we
can now get a voxel histogram by summing up the clusters with each of the PDs. The voxel
histogram is then convolved with the average neuronal tuning function (cosine tuning), to obtain
the estimated “tuning curve” of the voxel. Because of the assumed uniformity of the distribution
of PDs (Georgopoulos et al., 1988), the more clusters per voxel (i.e., smaller clusters), the flatter
the voxel's tuning curve. The voxels' CV is then calculated over 5 consecutive directions (of the
8 possible directions, mimicking our sampling in the fMRI experiment).
Figure 1: An example voxel with 16 clusters. Each of the 16 squares represents a
cluster with a PD according to the direction of the arrow. The PD of the entire voxel
is the vector sum of the cluster PDs.
This procedure was repeated 1542 times (an average of 140 voxels in each of the 11 participants)
with varying number of clusters per voxel (range: 5-1500 clusters), so that the average CV could
be calculated per cluster size. The average was calculated across voxels for each participant
separately, and then across participants, as was done for the real data. We then plotted the
average CV as a function of the number of simulated clusters per voxel. As can be seen in figure
7 of chapter 1 of the results, the smaller the cluster size (i.e., larger number of clusters per voxel),
the lower the average CV.
It is possible that neuronal tuning is in fact sharper than a cosine waveform (Amirikian and
Georgopulos, 2000). To find the lower bound on cluster size, the average voxel CV was similarly
23
calculated for various cluster sizes under the assumption that the average neuronal tuning curve
was narrower than 45° (thereby avoiding the smoothing effect imposed by the convolution
kernel).
Our goal was to find the cluster size that best corresponds to the mean CV of the actual data.
Rotation – experimental paradigm and data analysis
In the second study, after the first run of center-out reaching movements, the participants
practiced for ~10 min (inside the scanner) on the visuomotor rotation task, in which the cursor
was rotated by 450 CCW with respect to the hand movement. Thus, for the cursor to move
towards the target, participants had to move the joystick toward the neighboring (clockwise)
target without seeing their hand. The participants had adapted to this new mapping rule
implicitly. After the participants reached the targets successfully, they were scanned again while
carrying out another 250 “rotation” trials
After data preprocessing (as described in the methods of results - chapter 2), we calculated the
correlation coefficient (CC) between the columns of the baseline and the rotation run (each
corresponding to the pattern of activation across all voxels for a given condition). If the
estimated β weights contained information about the direction of movement, we expected to find
a higher CC for vectors that represent movements to the same direction (in the two datasets)
compared with the CC calculated for two different directions. Because the CCs were between
baseline movements and rotated cursor movements, we could distinguish between conditions that
shared the same hand movement (but differed in their visual aspects, i.e., target location and
cursor trajectory) and conditions that shared the same visual aspects (but differed in their hand
movement).
When the movements are aligned according to the direction of joystick movement (“movement
alignment”), the baseline and rotation run share the motor component but differ in their visual
components (target position and cursor direction). Similarly, during “target alignment”, the two
conditions share the same visual components but differ in their motor aspects (by 45°).
24
Now we need to find a control group of CCs to compare these correlations to, in order to find out
if they are significant. However, the angular difference between movement directions for the
same target alignment is small (450). As I have shown in the first results chapter, the CCs
between compared movements typically drop gradually with increased angular difference (see
results chapter 1, figure 4). Therefore, a 450 angle between the two movement directions could
produce significant CCs when compared to greater angular differences, such as 900 or 1350,
where CCs are typically negative, even if there is no "target" effect.
To that end, instead of comparing the alignment of same target directions to all other
comparisons (the rest of the CC matrix, see results chapter 2, figure 2), we compare that
alignment (blue diagonal in the CC matrix) only to the other CCs with the same absolute angular
difference (450, red diagonal). These other CCs serve as the "target control" group. Although the
movement directions in this group are 45 degrees apart, just as in the "same target" group, the
targets are 90 degrees apart. Thus, we dissociate the effect of having the same target and the
effect of having the same, or close, movement directions.
Similarly (although highly unlikely), a positive CC between same hand movements could result
from the fact that the two movements have small angular differences between target locations
(separated by only 450). To that end, we compare these CCs (green diagonal in the CC matrix) to
a control group of comparisons where the target directions are 450 apart, but movement
directions are 900 apart (purple diagonal).
The methods of analysis of the electrophysiological data will be described in the methods section
of chapter 3 of the results.
25
Results I:
Functional Organization of Human Motor Cortex: Directional
Selectivity for Movement
26
The Journal of Neuroscience, June 30, 2010 • 30(26):8897– 8905 • 8897
Behavioral/Systems/Cognitive
Functional Organization of Human Motor Cortex:
Directional Selectivity for Movement
Michal Eisenberg,1,2* Lior Shmuelof,1* Eilon Vaadia,2,3 and Ehud Zohary1,2
1
Department of Neurobiology, Life Sciences Institute and 2Interdisciplinary Center for Neural Computation, Hebrew University, Jerusalem 91904, Israel,
and 3Department of Physiology, Hadassah Medical School, Hebrew University, Jerusalem 91120, Israel
In monkeys, neurons in the hand representation of the primary motor cortex (M1) are often tuned to the direction of hand movement, and
there is evidence that these neurons are clustered according to their “preferred” direction of movement. However, this organizational
principle has yet to be demonstrated in M1 of humans. We conducted a functional magnetic resonance imaging (fMRI) study in which
participants used a joystick to move a cursor from a central origin to one of five equidistant targets. The fMRI signal of individual voxels
was sensitive to the directional aspects of the reaching task and manifested direction-specific adaptation. Furthermore, the correlation
between multivoxel patterns of responses for different movement directions depended on the angular distance between them. We
conclude that M1 neurons are likely to be organized in clusters according to their preferred direction, since only such a coarse-grained
representation can lead to directional selectivity of voxels, encompassing millions of neurons. A simple model that estimates cluster size
suggests that the diameter of these clusters is on the order of a few hundred micrometers.
Introduction
Neurons in the primary motor cortex (M1) of monkeys are
tuned to the direction of limb movement (Georgopoulos et al.,
1982). Single-cell recordings from M1 suggest that these neurons are organized according to their preferred directions (PDs)
(Asanuma and Rosén, 1972; Amirikian and Georgopoulos, 2003;
Ben-Shaul et al., 2003; Georgopoulos et al., 2007). Directional
tuning has also been found in the multiunit activity recorded
from M1 (Stark et al., 2009), as well as from local field potentials
(LFPs) (Mehring et al., 2003; Rickert et al., 2005), which represent populations at a resolution of ⬃1 mm (Berens et al., 2008;
Rasch et al., 2009). Together these data suggest that in monkeys,
M1 neurons are clustered, to some extent, according to their PDs.
This organizational feature resembles the columnar organization
characteristic of somatosensory cortex (Powell and Mountcastle,
1959), primary visual cortex (V1) (Hubel and Wiesel, 1962), middle
temporal cortex (MT) (Albright et al., 1984), and primary auditory
cortex (Imig and Adrian, 1977).
Obviously, little is known about the properties of neurons in
the homologous area (M1) of humans. A recent study, performed
for clinical purposes, showed that human M1 neurons are also
often directionally tuned (Truccolo et al., 2008). It is less clear,
Received Jan. 3, 2010; revised March 15, 2010; accepted March 26, 2010.
This research was supported by an Israel Science Foundation Grant 39/09 to E.Z. We thank I. Nelken, S. Wise, Y.
Pertzov, E. Stark, H. Sompolinski, and D. Shore for their insightful comments and Eldad Assa and Yuval Link for
programming the task.
*M.E. and L.S. contributed equally to this work.
Correspondence should be addressed to Michal Eisenberg, Department of Neurobiology, Life Sciences Institute
and Interdisciplinary Center for Neural Computation, Hebrew University, Jerusalem 91904, Israel. E-mail: michal.
[email protected].
L. Shmuelof’s present address: Motor Performance Lab, Neurology Department, Columbia University, New York,
NY 10032.
DOI:10.1523/JNEUROSCI.0007-10.2010
Copyright © 2010 the authors 0270-6474/10/308897-09$15.00/0
however, whether the neurons are organized in functionally related clusters, such that neighboring neurons share similar tuning
properties. To address these issues, we used functional imaging
techniques coupled with multivoxel pattern analysis. This approach seems, at first, unlikely to reveal functional clustering in
M1, if only because the spatial resolution of functional magnetic
resonance imaging (fMRI) (several millimeters) is much larger
than the size of functional units in the cortex. For example, the
typical diameter of a cortical column in primary visual cortex,
defined on the basis of orientation selectivity, measures hundreds
of micrometers in diameter (Berman et al., 1987). Nevertheless,
recent applications of multivoxel pattern analysis in imaging
studies allowed the detection of columnar organization in visual
areas such as V1 (Kamitani and Tong, 2005) and MT (Kamitani
and Tong, 2006). In this study, we use these analysis methods to
test the hypothesis that neurons cluster according to their directional preferences in the human M1 cortex. We reasoned that
examination of directional preferences at the voxel level could
support this hypothesis, provided that the number of clusters
within a voxel is small. In this case, random fluctuations in the
number of clusters with preference for a specific direction, together with the natural tuning characteristics of the neurons,
might determine the directional preference of the voxel as a
whole.
To that end, our participants performed a “center-out
task,” similar to the one performed by monkeys in studies of
M1 (Georgopoulos et al., 1982). During an event-related fMRI
scan, our participants repeatedly moved a cursor from the center
of a screen toward various targets in the periphery by moving a
joystick in the corresponding direction. We found (1) that M1
voxels were selective for the directional aspects of the reaching
task, (2) that the patterns of activation across M1 voxels became
less correlated as the angular difference between movements in-
8898 • J. Neurosci., June 30, 2010 • 30(26):8897– 8905
Eisenberg et al. • Functional Organization of Motor Cortex
creased, and (3) that direction-specific
fMRI adaptation [i.e., repetition suppression (RS)] occurred when the same movement was repeated in the following trial.
These results support the hypothesis that
human M1 is organized in clusters of neuronal populations with similar PDs of
movement.
Materials and Methods
Participants. Eleven right-handed volunteers
with normal or corrected-to-normal visual
acuity and no neurological or psychiatric history (5 women and 6 men, aged 18 –35) participated in the present experiments. Hadassah
Ein Kerem Medical Center Ethics Committee
approved the experimental procedure. Written
informed consent was obtained from each
participant.
MRI acquisition. The blood oxygenation
level-dependent (BOLD) fMRI measurements
were performed in a whole-body 3T Trio Siemens scanner. The functional MRI protocols
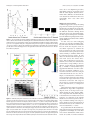
were based on a multislice gradient echo- Figure 1. Experimental design and movement trajectories. a, Trial flow (left to right): each trial started with the presentation
planar imaging and a standard head coil. The of a red circle at the center of a screen (“origin”). Then, 0.5 s later, five circles appeared in the periphery, four blue circles and one
functional data were obtained under the opti- green circle, which indicated the future movement target position (“target”). The red “origin” changed its color to white 2 s later,
mal timing parameters: TR ⫽ 2000 ms, TE ⫽ instructing the participant to move the cursor (the white circle) toward the target (green circle). The participant had 1.5 s to
30 ms, flip angle ⫽ 90°, imaging matrix ⫽ 80 ⫻ complete the movement. Movements in five directions were counterbalanced across trials. b, Example of trajectories in all five
80, voxel size: 2.75 ⫻ 2.75 ⫻ 3.1 mm. The 30 directions for one of the participants.
slices (with a gap of 0.3 mm) were oriented in
the axial direction. The scan covered the whole
10,000 random sequences sampled. The experiment began and ended
brain. Each participant was scanned in one run lasting 20.5 min. The run
with 16 s of a null event.
was comprised of an acquisition of 616 volumes and contained 250 trials.
We used only five directions of movement, covering only half of the
Experimental paradigm. Each trial lasted 4 s. The trial began with preplane, instead of eight targets covering the entire plane, to achieve as
sentation of a red circle in the center of the screen (“origin,” radius of
many trials as possible for each condition to obtain a reliable signal. We
0.7°). Initially, the participants had to hold an MRI-compatible joystick
also chose to use targets with rather small angular differences (45°) rather
still, and make no hand movement. After an interval of 500 ms, five
than spanning the entire plane with larger differences (72°). This was
circles (targets, radius of 1°) appeared at the upper half of the screen,
done to allow us to assess not only the selectivity for a specific direction of
spread around the center at equal distances, between 0° and 180°, 45°
movement, but also the relationship between voxel representations for
apart. Their distance from the origin was 4.5° of visual angle (Fig. 1). Four
similar movements (to neighboring targets).
of the circles were blue and one circle was green, signaling the required
Data analysis. Preprocessing and defining regions of interest (ROIs)
future direction of movement. The participants had to keep their hand
was done using Brain Voyager QX (Brain Innovation).
still until the “go” signal, in which the red “origin” circle turned into a
The functional images were superimposed on two-dimensional anawhite cursor, which occurred 2 s later. They were instructed to respond
tomical images and incorporated into the three-dimensional datasets
by moving the cursor toward the green target, using the joystick (Fig. 1a).
through trilinear interpolation. Before statistical analysis, head motion
Participants had 1.5 s to reach the target. Upon reaching the target, all
correction and high-pass temporal filtering in the frequency domain
circles disappeared. This served as a cue for the participants to release the
(3 cycles/total scan time) were applied to remove drifts and to imjoystick (thereby relaxing the spring), which resulted in the joystick reprove the signal-to-noise ratio. The complete dataset was transturning to its starting position at the center. There was no explicit failure
formed into three-dimensional Talairach space with a resolution of
signal. If the participants did not reach the target within the 1.5 s time
3 ⫻ 3 ⫻ 3 mm3.
limit, all circles disappeared and the next trial began (participants failed
The left M1 ROI was individually defined for each participant as a
to reach the target in 10.6% of the trials). There were also intermittent
cluster within the central sulcus, which showed higher activation during
null trials in which the red origin and all five blue targets appeared on the
movement than during rest (general linear model, p ⬍ 10 ⫺8; Bonferroni
screen for 2– 8 s (thus no target was distinctly marked for movement).
corrected, p ⬍ 0.01). The average size of M1 was 140 ⫾ 40 functional
These trials were obviously not followed by a “go” signal. During these
voxels (see supplemental Table 1, available at www.jneurosci.org as supnull trials, the participants were instructed not to move the joystick until
plemental material).
the next trial began. The task was programmed with MATLAB version 7.1
Further analysis was done using Matlab R2007b (MathWorks). We
(MathWorks), using Psychtoolbox (Brainard, 1997; Pelli, 1997).
used linear regression to estimate response amplitudes ( values) for
This rapid event-related fMRI study consisted of 250 trials, 50 in each
each functional voxel in each condition, solving an equation of the form
direction. The trial order was counterbalanced (first order) and embedy ⫽ Xb ⫹ e, where the vector y is the measured voxel time course, the
ded with null trials of various lengths (2, 4, 6, or 8 s). The different lengths
vector b contains a sequence of the estimated response amplitudes for
of the null trials were used to randomize the timing of the movement
each of the five conditions, X is the convolution matrix determined by
trials, which allow a more efficient estimation of activation (Dale, 1999).
the sequence of events, and e is the error (Gaussian noise). The convoluNull trials were pseudorandomly embedded between movement trials.
tion matrix, X, was designed with predictors of onset times for each trial
Our constraint was that the total length of all null trials would equal the
and has the dimensions of 614 ⫻ 51. X contains a row for each time point
total length of each of the five movement conditions (200 s). The trial
(TR) in the experiment (totaling 614) and 10 columns for each of the 5
sequences were built using optseq software, which chooses the most
conditions (or conditions in the correlation analyses) plus a column of
efficient sequence (most variable history before each condition) out of
Eisenberg et al. • Functional Organization of Motor Cortex
J. Neurosci., June 30, 2010 • 30(26):8897– 8905 • 8899
ones for the offset predictor (thus 51 columns). This leads to an independent estimation of the response amplitude, for each condition at each
time point (the first point out of the 10 points for each condition is the
time of the beginning of a trial). The vector of response amplitudes, b, is
estimated using the equation b ⫽ (XⴕX )⫺1Xⴕy and is respectively comprised of 10 values (estimated  values) for each condition (one for each
time point between 0 and 18 s after the beginning of the trial). The first
value of b is the mean activation over the entire time course. Importantly,
since we estimated the BOLD activation for each time point separately,
no assumptions were made about the shape of the hemodynamic response. Linear regression was significant in ⬎99% of voxels. On average,
the variance explained by the model accounted for 37% of the total
variance.
Coefficient of variation analysis. We used a bootstrap analysis, to test
whether the coefficient of variation (CV) (SD/mean) of the five directions of movement in each voxel is significantly higher than expected by
mere chance (e.g., had there been no directional selectivity). In this analysis, we randomly reassigned conditions (directions of movement) to the
trials, while maintaining the original proportions of hand movements
(each condition was assigned to 20% of the trials). The null conditions
were not replaced. We created a new regression matrix, estimated the 
values for the different directions, and assessed the CV, separately for
each assignment. This procedure was repeated 10,000 times, resulting in
a distribution of expected CVs merely due to noise. Next, for each voxel,
we assessed its p value: the fraction of CV values obtained by the bootstrap method that were greater than the actual CV of that voxel. Finally,
a 2 test was used to show that the distribution of p values across voxels
(shown in supplemental Fig. 3, available at www.jneurosci.org as supplemental material) was significantly skewed to low values (differing from a
uniform distribution, which would be expected by chance).
The CV analysis was used also for the estimation of cluster size (see
Discussion and Fig. 7). For this purpose, since each voxel is expected to
show some variation in its response by chance (as there were limited
repetitions of each direction of movement), we also corrected each voxel’s
CV by subtracting from its actual variance in response (for the various
directions) the mean variance of the bootstrapped activation (XBS) across
iterations:
CV ⫽
冑 (VarB ⫺ mean (VarBBS))
mean共B)
.
In addition to calculating voxel CV, we also calculated the CV as a
measure of the directionality of the LFPs in monkey M1, when monkeys performed the same center-out task [courtesy of S. Cardoso de
Oliveira (Cardoso de Oliveira et al., 2001)]. To that end, we used the
peak-to-peak distance (the distance between maximum and minimum) of the mean evoked potential, per direction of movement. CVs
of LFPs were calculated without subtracting bootstrapped variance.
Consequently, the CV is slightly overestimated.
Analysis of spatial patterns of fMRI response. To detect directional selectivity of voxel population spatial patterns, each individual’s data were
split into two datasets, such that each of the 50 trials in each direction was
randomly assigned to one of the datasets. For each voxel, we estimated
the  values and defined the activation for each direction (i.e., activation
value) as the average of the  values measured 6 and 8 s after the beginning of the trial (⬃4 – 6 s after movement initiation; normally at the peak
activation) (see Fig. 2b). Then, in each dataset and for each voxel, we
subtracted the voxel’s mean activation level (across all directions) to
remove activation differences between voxels that are unrelated to movement direction. Without such a correction procedure, one would get
high correlations between the multivoxel spatial patterns from all comparisons, simply because some voxels are more active than others, regardless of the direction of movement.
The above analysis resulted in two matrices, one for each dataset. Each
matrix consisted of 5 columns (one for each direction) with length N equal to
the number of voxels in the ROI. The entries of each row of the matrix were
the 5 activation values of a single voxel for all 5 directions of movement.
Next, we calculated the correlation coefficient (CC) between the columns of the first and the second datasets (each corresponding to the
pattern of activation across all voxels, for a given condition). If the activation values contain information about the direction of movement, one
should get a higher CC for movements to the same direction (in the two
datasets) than the CC calculated for movements to two different directions. To address the concern of low-frequency temporal trends in our
data, we repeated this analysis 100 times for each participant; in each
iteration, the data were split differently into two random datasets. The
results shown are the mean CC values across all 11 participants. The resulting
dependence of the CC on the angular difference between the two directions
of movements matched a normal distribution:
yi⫽h ⫻ e⫺
(xi ⫺ )2
2
⫺ b.
Analysis of spatial patterns of simulated data. Individual maps often
display a preference for some directions over others. In other words, the
distribution of preferred directions across voxels was not uniform
(though the identity of the most common preferred direction varied
among participants). This naturally raises the concern that the information about movement direction could potentially be due to artifacts at a
coarse scale (such as large blood vessels, which may affect the activation
of many voxels). To address this concern, we performed a new analysis in
which we used simulated data. Simulation of the data was done by replacing the activation values for each direction (across voxels) with random values taken from a normal distribution with the same mean and SD
as the real data for that direction. Now tuning of voxels is due only to the
global bias of one direction over others in the entire ROI. We ran the
multivoxel pattern analysis with the simulated data. Tuning of the multivoxel pattern would imply that our results do not stem from any organization at the subvoxel level, but rather from a large-scale variability
between directions (Op de Beeck, 2010). See supplemental Figure 2
(available at www.jneurosci.org as supplemental material) for results.
Movement repetition analysis. In the repetition analysis, the various
conditions were also split according to the angular difference between the
direction of movement in the current trial and the movement direction
in the previous trial. Thus, a condition of 0° means that the movement
was to the same direction as in the previous trial. Beta values were estimated in the same way as in the previous analyses.
To test whether a voxel’s PD (direction eliciting the greatest response)
is the same direction that elicits the greatest repetition suppression (RS),
we divided the trials into 10 conditions. First we divided the trials into
repeated and nonrepeated trials, and then we divided each set of trials
into five conditions according to the direction of movement. The repetition index for each voxel (v) and each direction (d) was defined as
v,d(nonrepeated)/v,d(repeated).
Both repetition index and activation index (of nonrepeated trials)
were normalized by subtracting the mean and dividing by the SD across
directions in each voxel to bring the two different signals to a common
scale and then correlated. Pairwise Student’s t test was used to test
whether the CCs in the “same direction” and “different direction” conditions were significantly different.
Results
Participants performed a “center-out” task in a rapid eventrelated fMRI experiment. In this task, they had to move a cursor
from the center of a video screen toward one of five equidistant
targets located at the periphery of the screen. On each trial, one of
the five targets had a different color than the rest, to indicate that
it was to be the future target, and the participants later used an
MRI-compatible joystick to move the cursor to that location on
the screen (Fig. 1a). They made a series of center-out movement
trials in the five different directions while being scanned. Null
trials (with no movement) were also embedded in the scan pseudorandomly. Each movement was performed 50 times in a counterbalanced order during the course of a 20.5 min scan. The
participants reached the target correctly on 89.4% of the trials.
The angular deviation of movement was assessed for each trial by
calculating the angle between the actual trajectory at the peak
8900 • J. Neurosci., June 30, 2010 • 30(26):8897– 8905
Eisenberg et al. • Functional Organization of Motor Cortex
Figure 2. Extraction of the hemodynamic response kernels for the different movement directions. a, The time course of activation of an example voxel. Each color denotes movement in one of
the five directions according to the arrows on the right. The direction of movement was randomized across trials, interspersed with null (no-movement) trials (in gray). In this example only the first
240 s of the session are shown. b, The extracted hemodynamic response kernel for each movement direction (using the deconvolution method). Colors correspond to the movement directions
depicted in a. For each direction, the mean activation level (estimated  value) between the two dashed lines was used as the voxel’s activation value for further analysis. c, Definition of left M1.
ROIs were defined for each participant individually as a cluster of voxels within the central sulcus of the left hemisphere, which showed higher activation during movement than during rest ( p ⬍
10 ⫺8; Bonferroni corrected, p ⬍ 0.01).
velocity and the direct trajectory to the center of the target. The
mean absolute angular deviation was 10.4° (Fig. 1b).
We defined cortical regions involved in the visuomotor reaching task by identifying voxel clusters that exhibited significant
enhanced activity during the task compared with null trials ( p ⬍
10 ⫺8; Bonferroni corrected at least p ⬍ 0.01). Several ROIs were
defined for each participant, but here we focus on the results from
left M1 (Fig. 2c).
The resulting time course of activation for each voxel, acquired during the scan, was further analyzed using linear regression to estimate the profile of activation generated by each of the
five directions of movements. An example of a voxel’s time
course of activation and the extracted convolution kernels (hemodynamic response) to the different directions are shown in
Figure 2. For each voxel, the average of the estimated  values
measured 6 and 8 s after the beginning of the trial (which were
usually the time points of peak activation) served as the extracted
parameter of activation level, for each direction.
Voxel tuning analysis
We began by inspecting the degree of directional tuning in each
voxel. Note that reaching movements were made only to targets
in the upper half of the screen (corresponding to forward joystick
movements). Therefore, we could not use the classical estimation
of tuning, i.e., cosine fits (Georgopoulos et al., 1982), and had to
use other measures instead. For each voxel, we computed the CV,
which is the SD of the voxel’s activation level (across different
directions), divided by its mean activation level (across those
directions). Next, we assessed the probability that each of the
voxels would exhibit directional specificity simply by chance, by
conducting a bootstrap test in which the direction of each movement was randomly reassigned (in the regression matrix). This
analysis revealed that 28% of the voxels in M1 were directionally
selective (i.e., they had a CV greater than 95% of the CV values
obtained by the bootstrap method). A 2 test showed that the
distribution of p values across voxels (supplemental Fig. 3,
available at www.jneurosci.org as supplemental material) was
significantly skewed to lower values from those expected by
2
chance ((9)
⫽ 1386; n ⫽ 1542; p ⬍ 0.001). Note also that since
not all directions of movement were tested, this percentage is
likely to be a lower bound on the percentage of voxels sensitive
to movement direction.
Next, we aligned all the voxels’ activation tuning curves according to their PD to obtain an average voxel tuning curve (Fig.
3). This computation allowed us to assess whether the average
voxel’s preference is only for a single, specific PD or whether there
is instead gradual tuning at the voxel level. In the latter case,
movement in directions close to the PD, in angular terms, should
elicit a greater BOLD response than movements far from the PD.
Figure 3b shows that the BOLD signal decreases with angular
distance from the voxel’s PD. A one-way repeated-measures
ANOVA with direction difference as the relevant factor, taking
into account only the BOLD signal for movements with an angular difference of 45°, 90°, and 135° apart, indicated that this factor
was marginally significant (F(2,20) ⫽ 3.29, p ⬍ 0.058) (movements with a 0° difference were discarded from this analysis be-
Eisenberg et al. • Functional Organization of Motor Cortex
J. Neurosci., June 30, 2010 • 30(26):8897– 8905 • 8901
cause, due to our alignment procedure,
their BOLD signal is necessarily greater
than the other angular distances; movements with a difference of 180° from PD
were discarded as well because there were
considerably fewer cases than other
directions).
Figure 3. M1 voxels show directional tuning. a, Example of individual voxel “tuning curves” showing the BOLD activation of 3
voxels as a function of movement direction. Each tuning curve was normalized such that the mean activation of each voxel across
all directions was zero. In each participant, the “tuning curves” of all individual voxels from M1 were aligned according to their PD,
to generate a “voxel population tuning curve.” This population tuning curve was averaged across participants. b, The resulting
“average voxel” activation as a function of the angular distance from the PD. The peak at the PD is a direct result of the alignment
procedure, but note the smooth tuning preference as the angular distance between directions of movement increases. Error bars
denote SEM across participants.
Figure 4. Multivoxel spatial patterns of activation are more similar for closer movements. a, Example of the patterns of M1
activation in one subject for movements in 90° (top) and 180° (bottom), in the two datasets. These examples (shown mainly for
illustrative purposes) are taken from the axial plane (Talairach coordinates: z ⫽ 50) from one of the participants. Blue arrows
denote correlations between movements in the same directions (0.33 and 0.56 for this participant), whereas red arrows denote
correlations between movement in the different directions (⫺0.43 and ⫺0.31). b, Matrix of the average CCs between the
multivoxel patterns in the two datasets (across all participants), for all possible direction combinations. The main diagonal corresponds to CCs between same directions; the next diagonals (in gray) correspond to the CC between directions 45° apart, etc (in
lighter colors). Red and blue values correspond to the average value (across participants) for the directions in the example
(a). c, The similarity between patterns of voxel activations (measured by the CC) decreases as a function of the angular distance
between the two directions of movements. The grayscale of each data point (diamonds) indicates the appropriate diagonal in b
whose average value is depicted. Error bars denote SEM across participants. The black curve denotes the Gaussian fit to the CCs.
Multivoxel pattern analysis
A different approach to study the tuning
properties of voxels in a given region is to
calculate the correlation between the multivoxel spatial patterns of activation for
the different directions. Having shown
that voxels show some directional tuning,
one may expect to find greater correlation
between multivoxel spatial patterns of activations during movements with a smaller
angular difference.
To that end, two non-overlapping
datasets were created by randomly assigning each of the 50 trials in each direction
into one of the two categories. Next, we
used linear regression to estimate the
level of activation of each voxel in each
movement direction separately for each
dataset. Finally, to eliminate differences
in activation between voxels, in each
dataset we subtracted the mean response of
each voxel (across the various directions). This resulted in two vectors of activation (across voxels) for each direction
of movement, corresponding to the two
datasets (see PD maps in supplemental
Fig. 1, available at www.jneurosci.org as
supplemental material). We then calculated the correlation coefficient (r) between the activation vectors for each pair
of directions from the two sets. (For example, we calculated the correlation between activation elicited by movements
toward the 90° target in the first set and
movements toward the 180° target in
the second set, or by movements elicited
in the same direction; see examples in
Fig. 4a.)
This resulted in a matrix of correlation
coefficients for all possible comparisons
(Fig. 4b). Next, we averaged across conditions having the same angular difference (diagonals of the matrix in Fig. 4b)
to obtain the mean correlation (between
patterns) as a function of the angular
distance between the trajectories: the average correlation tuning curve (Fig. 4c).
As the distance between the directions of
movement increased, the correlation decreased (Gaussian fit, r ⫽ 0.999).
It is possible that the directional selectivity at the level of representation of voxel
populations could simply be a side effect
of an uneven distribution of PDs across
voxels. To test this, we ran the correlation
8902 • J. Neurosci., June 30, 2010 • 30(26):8897– 8905
Eisenberg et al. • Functional Organization of Motor Cortex
analysis using simulated data that preserved the original uneven distribution
across voxels. We found no tuning of the
correlation coefficients of the patterns of
activation for the simulated data (supplemental Fig. 2, available at www.jneurosci.
org as supplemental material). We conclude that our results reflect information
based on the direction tuning at the voxel
level, rather than a global bias in preference toward some directions over others.
Figure 5. RS of the fMRI response in primary motor cortex. a, The various colors denote the mean time course of activation in the
Effect of movement RS
Another way to study the selectivity of M1 ROI (across participants) for a movement in a given direction when preceded by a movement in the same direction in the
BOLD activation to the direction of move- previous trial (blue line) or a different direction (green, red, and cyan denote cases in which the previous trial was 45°, 90°, and 135°
ment is to examine the effect of movement apart from the current trial, respectively). Suppression is seen only for cases of repetition of the same movement. b, The mean level
of activation (measured at time points 6 and 8 s and then normalized such that the mean of each voxel across directions was 0) as
repetition. RS, the reduction in the BOLD a function of the absolute angular difference between the current movement and the movement in the previous trial. Colors
signal following repeated presentation of correspond to the conditions shown in a. Error bars denote SEM between participants.
the same stimulus, was initially reported in
visual cortex (Grill-Spector and Malach,
2001). It was recently documented for
motor actions as well (Dinstein et al.,
2008). One might therefore expect to find
greater reduction of the BOLD signal in
M1 when the same movement is repeated
across trials. To test this, we categorized
the trials according to their similarity to
the previous trial. Figure 5 shows the average time course of activation across M1
voxels (Fig. 5a) and the mean BOLD activation (Fig. 5b) as a function of the angular difference from the previous trial. The
BOLD activation is lower when the participant made a movement toward the same
target as in the previous trial (one-way
repeated-measures ANOVA, F(4,40) ⫽ 5.58,
p ⬍ 0.005, Tukey–Kramer post hoc test,
p ⬍ 0.005). This phenomenon seems to be
limited to the same direction, and does
not generalize to near targets (i.e., there is
no statistical difference in the BOLD activation for the current movement when the
previous movement diverged from it by
45°, 90°, or 135°, F(3,30) ⫽ 0.4, p ⫽ 0.75).
Is the preferred direction of the voxel
(eliciting the greatest response) also the
direction that elicits the greatest RS? If so,
repeated movements toward the voxel’s
Figure 6. RS multivoxel patterns are correlated with the activation patterns. a, Example of the patterns of activation (left) and
PD (direction with maximum activation) the repetition index across voxels (right) for movement at 45° (top) and 90° (bottom). These examples are taken from the axial
should lead to greater RS than repeated plane (Talairach coordinates: z ⫽ 50) of one of the participants. Blue arrows denote correlations between the two measures for the
movements to other directions. To that end, same movement (0.59 and 0.61, respectively for the participant in the example), whereas red arrows denote correlations between
we assessed the correlation between the pat- the two measures for different movements (⫺0.26 and ⫺0.09 for this participant). b, The resulting matrix of CCs when comparing
tern of multivoxel activation for a given di- the patterns of activation and the repetition index in all possible directions of movement. The main diagonal corresponds to CCs
rection and the pattern of multivoxel RS for between same directions.
that direction, as well as for all other direccomputation of both measures, the nonrepeated trials were
tions (see example for the 45° and 90° movement cases in Fig. 6a).
divided into two separate groups: one for computing the repThis resulted in a matrix of correlation coefficients between the repetition index, the other for the activation index. Our results
etition suppression and activation level for all possible movement
remained significant (t(10) ⫽ 5.01, p ⬍ 0.001).
pairs. Our findings show that the level of activation and RS are posWe further tried to assess, on a voxel-by-voxel basis, whether the
itively correlated for same directions (mean CC ⫽ 0.29, SD ⫽ 0.09),
direction of movement similarly affected both the voxel’s level of
and significantly greater than different directions (mean CC ⫽
activation and its repetition index. Had the two measures been un⫺0.07, SD ⫽ 0.02; t(10) ⫽ 10.12, p ⬍ 5 ⫻ 10 ⫺6). To verify that
our results did not stem merely from use of the same data for
correlated, one might expect that 20% of the voxels would show that
Eisenberg et al. • Functional Organization of Motor Cortex
J. Neurosci., June 30, 2010 • 30(26):8897– 8905 • 8903
(Amirikian and Georgopoulos, 2003;
Ben-Shaul et al., 2003; Stark et al.,
2009).
RS
We find clear evidence for RS in human
M1. This finding provides indirect evidence that the direction of movement is a
key factor determining the response of
neurons in the human motor cortex. A
voxel’s direction tuning is determined by
two factors: the distribution of PDs across
neuronal populations within the voxel
and the average neuronal tuning width.
Anisotropy of the distribution of PDs
(due to the coarse-grained clustering of
neuronal populations with similar PDs
within a voxel) would result in direction
preference at the voxel level. The most
prevalent neuronal PD within the voxel’s
population of neurons will determine the
voxel’s PD. The voxel’s tuning width,
however, is affected both by the average
neuronal tuning width and, possibly, by
Figure 7. Estimating the number of direction-selective clusters within a human M1 voxel. a, A voxel is modeled as having either
spatial correlations (i.e., greater tendency
10 such clusters (left) or 100 (right). The resulting distribution of their PD within a voxel (assuming a random choice from a uniform
of a voxel to contain more neurons with
distribution with 8 possible PDs) is then convolved with the average neuronal tuning function (cosine tuning), to obtain the
estimated “tuning curve” of the voxel. The voxels’ CV is then calculated using 5 consecutive directions (of the 8 possible directions, PDs close to the most prevalent PD than
mimicking our sampling in the fMRI experiment). b, This procedure was repeated for all possible cluster sizes, resulting in a function neurons with far PDs).
In principle, RS may be helpful in disdescribing the mean CV as a function of number of clusters in each voxel (blue curve). Similarly, the expected voxel CV was
calculated for various cluster sizes, under the assumption that the average neuronal tuning curve was narrower than 45° (thereby tinguishing between the neuronal tuning
avoiding the smoothing effect imposed by the convolution kernel; red curve). The black horizontal line denotes the mean CV characteristics of the voxel population
obtained across all participants’ M1 voxels, and the gray shading denotes the mean CV ⫾ SD. The points of intersection, corre- and the spatial distribution of clusters, as
sponding to the estimated number of clusters in a voxel, are 42 and 1208 clusters, for the two assumed neural population tuning the degree of RS is thought to be related to
characteristics, respectively.
the average properties of single neurons
(Grill-Spector and Malach, 2001). We
find that the repetition effect is narrower
than 45°; a decrease in activation is seen
the repetition index was maximal at the voxel’s PD. We found that
only for a movement with the same direction as in the previous
this was the case in 30% of the voxels, significantly more than that
trial, while a movement 45° away from the previous one does not
expected by chance (t(10) ⫽ 4.52, p ⬍ 0.0005).
lead to any significant activation suppression. In this case, we
Discussion
would conclude that the tuning width of the voxel is determined
In this study, we show that M1 voxels are directionally tuned.
mostly by spatial correlations. However, this finding is at odds with
This finding, at first, seems puzzling: based on an estimation of
the broad tuning of neurons in macaque M1, which are well fit by
⬃50,000 neurons in 1 mm 3 of cortex (Beaulieu and Colonnier,
a cosine waveform. It is possible that in human M1 the average
1983), there are on the order of 1,000,000 neurons in each voxel.
neuronal tuning curves are narrower than in primates, as has
If neuronal PDs are uniformly distributed across the population,
been recently found for frequency tuning in auditory cortex
one would expect that any fluctuations in firing rates would av(Bitterman et al., 2008). However, an alternative interpretaerage out, and the overall activity during different movements
tion is that the relationship between RS and single-cell propwould be the same. Thus, no directional preference should be
erties for actions is more complicated than the models so far
seen at the voxel level.
proposed for sensory inputs (Grill-Spector et al., 2006). It is
The finding that voxels do show selectivity to direction of
also possible that the RS could reflect the tuning properties of
movement suggests that the spatial distribution of the neurothe inputs from other areas (Sawamura et al., 2006), and that
nal PDs is not random. If neurons sharing the same PD are
the narrower width of the RS effect is a reflection of the narclustered in a columnar fashion, a voxel should contain many
rower tuning curves in visual or parietal areas or their limited
fewer independent elements. Under such circumstances, direceptive field size (Heggelund and Albus, 1978; Andersen et al.,
rectional preference at the voxel level may not be that surpris1985).
ing. A similar preference of voxels in the human cortex has
been seen in areas with known columnar organization in the
Estimating cluster size
monkey, such as V1 (for orientation) (Kamitani and Tong,
One central goal in this paper was to roughly estimate the size of
2005) and MT (for direction of motion) (Kamitani and Tong,
the average direction-selective cluster in M1 from our data. Con2006). Our results are also consistent with electrophysiologisequently, we constructed a model with some simplifying ascal evidence in monkeys suggesting some degree of clustering
sumptions. We assumed that M1 neurons have preference for
of neurons in M1, based on their direction selectivity
one of eight discrete directions (which differ by 45°), and the
Eisenberg et al. • Functional Organization of Motor Cortex
8904 • J. Neurosci., June 30, 2010 • 30(26):8897– 8905
direction tuning curve can be approximated by a cosine function
[in which the mean baseline activity and modulation index were
taken from Georgopoulos et al. (1982)]. These neuronal parameters were used to describe the tuning properties of an entire
cluster, since a cluster was assumed to contain only neurons with
the same PD. We also assumed that the PDs of the different
clusters are uniformly distributed. However, as the number of
clusters per voxel becomes smaller, random picks from this uniform distribution are likely to generate an uneven number of
clusters tuned to each of the eight possible directions (Fig. 7a).
This would lead to a preference for a specific direction at the voxel
level, which would be smoothed to an extent by the characteristic
tuning function of the neurons. One can therefore calculate the
mean CV of each voxel as a function of the number of clusters
within that voxel. The CV thus provides a measure of the modulation in the voxel’s activation for the different directions, as
depicted in Figure 7a for the cases in which there were 10 or 100
clusters in a voxel.
Note that the average voxel’s CV drops sharply as the number
of direction-tuned clusters increases (Fig. 7b, blue and red
curves). The voxel’s CV, however, also depends on the average
neuronal tuning width. If tuning is wide (e.g., cosine tuning, blue
curve), the neurons also respond to other directions besides the
PD. This will obviously dampen variation in firing rates caused by
a nonhomogeneous distribution of PDs. When the average neuronal tuning width was taken to follow a cosine fit, the value
closest to the mean CV obtained in our experiment was ⬃40
clusters per voxel.
It is possible that neuronal tuning is in fact sharper than a
cosine waveform (Amirikian and Georgopulos, 2000). To find
the lower bound on cluster size, we recalculated the expected
voxel CV as a function of the cluster number assuming an
extremely narrow neuronal tuning (⬍45°) (Fig. 7b, red curve).
In this case, ⬃1200 direction-selective clusters within a voxel
are necessary to account for the variation observed in our
fMRI data.
It should be taken into consideration that our model assumes
no correlation between the tuning curves of neighboring clusters.
Accounting for such correlations might lead to an estimation of a
smaller cluster size. Notwithstanding these limitations, our estimates offer an upper and lower bound (at least in terms of orders
of magnitude) on the size of an individual direction-selective
cluster within human M1. If we further assume that the same PD
is represented across all cortical layers (Hubel and Wiesel, 1962)
and that the human cortex is ⬃2.5 mm thick, one can ignore the
third (depth) dimension of our 3 ⫻ 3 ⫻ 3 mm 3 voxels. Thus, in
the 3 ⫻ 3 mm 2 of voxel surface area, there are ⬃40 clusters per
voxel, which translate to a cluster diameter of ⬃470 m. On the
other hand, 1200 clusters per voxel (assuming a narrow tuning
curve) would indicate that cluster diameter is ⬃85 m.
Interestingly, a similar estimation is found when using tuning curves of the average LFP elicited by movements in eight
directions in monkey M1 (de Oliveira et al., 2001) (see Materials and Methods). The CV in this case was 0.2, which corresponded to 7 or 185 clusters per voxel [assuming cosine or
narrow (⬍45°) neuronal tuning width, respectively]. Assuming the LFP covers a surface area of 1 mm 2 (Berens et al., 2008;
Rasch et al., 2009), cluster diameter is estimated to be 75–380
m. Our estimation of the human M1 cluster diameter, in the
range of 85– 470 m, nicely concurs with the order of magnitude of cluster size in the monkey estimated here (using the
above LFP measures) as well as others (Amirikian and Georgopoulos, 2003; Stark et al., 2009).
Finally, it is important to remember that other components in
this study, besides the direction of hand movement, could have
influenced our results. These include the visual effects of target
location, eye movements (which are likely to vary according to
the target position), or visual aspects of the cursor movement.
Therefore, from our current results we cannot unequivocally ascertain whether the neuronal selectivity is due to limb movement
direction per se, or perhaps visual (or oculomotor) aspects of
directionality. We shall refer to these important issues, in depth,
elsewhere. It is also impossible to distinguish whether the reported activation is due mostly to neuronal activity during movement preparation (“Hold” period) or during the movement
itself. This issue requires further investigation.
Conclusions
We show that the fMRI signal of individual voxels in human
M1 is sensitive to the directional aspects of the reaching task
and manifests direction-specific adaptation. Furthermore, the
spatial patterns of the fMRI response are more correlated as
movement directions are closer. We conclude that human M1
neurons are organized in clusters according to their PD. The
model we constructed to estimate cluster size suggests that
cluster diameter is likely to be of the order of a few hundred
micrometers, which resembles the diameter estimated in
monkeys.
References
Albright TD, Desimone R, Gross CG (1984) Columnar organization of directionally selective cells in visual area MT of the macaque. J Neurophysiol 51:16 –31.
Amirikian B, Georgopoulos AP (2000) Directional tuning profiles of motor
cortical cells. Neurosci Res 36:73–79.
Amirikian B, Georgopoulos AP (2003) Modular organization of directionally tuned cells in the motor cortex: is there a short-range order? Proc Natl
Acad Sci U S A 100:12474 –12479.
Andersen RA, Essick GK, Siegel RM (1985) Encoding of spatial location by
posterior parietal neurons. Science 230:456 – 458.
Asanuma H, Rosén I (1972) Topographical organization of cortical efferent
zones projecting to distal forelimb muscles in the monkey. Exp Brain Res
14:243–256.
Beaulieu C, Colonnier M (1983) The number of neurons in the different
laminae of the binocular and monocular regions of area-17 in the cat.
J Comp Neurol 217:337–344.
Ben-Shaul Y, Stark E, Asher I, Drori R, Nadasdy Z, Abeles M (2003) Dynamical organization of directional tuning in the primate premotor and primary motor cortex. J Neurophysiol 89:1136 –1142.
Berens P, Keliris GA, Ecker AS, Logothetis NK, Tolias AS (2008) Feature
selectivity of the gamma-band of the local field potential in primate primary visual cortex. Front Neurosci 2:199 –207.
Berman NEJ, Wilkes ME, Payne BR (1987) Organization of orientation and
direction selectivity in areas 17 and 18 of cat cerebral cortex. J Neurophysiol 58:676 – 699.
Bitterman Y, Mukamel R, Malach R, Fried I, Nelken I (2008) Ultra-fine
frequency tuning revealed in single neurons of human auditory cortex.
Nature 451:197–201.
Brainard DH (1997) The Psychophysics Toolbox. Spat Vis 10:433– 436.
Cardoso de Oliveira S, Gribova A, Donchin O, Bergman H, Vaadia E (2001)
Neural interactions between motor cortical hemispheres during bimanual and unimanual arm movements. Eur J Neurosci 14:1881–1896.
Dale AM (1999) Optimal experimental design for event-related fMRI. Hum
Brain Mapp 8:109 –114.
Dinstein I, Gardner JL, Jazayeri M, Heeger DJ (2008) Executed and observed movements have different distributed representations in human
aIPS. J Neurosci 28:11231–11239.
Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT (1982) On the relations between the direction of two-dimensional arm movements and cell
discharge in primate motor cortex. J Neurosci 2:1527–1537.
Georgopoulos AP, Merchant H, Naselaris T, Amirikian B (2007) Mapping
Eisenberg et al. • Functional Organization of Motor Cortex
of the preferred direction in the motor cortex. Proc Natl Acad Sci U S A
104:11068 –11072.
Grill-Spector K, Malach R (2001) fMR-adaptation: a tool for studying the
functional properties of human cortical neurons. Acta Psychologica
107:293–321.
Grill-Spector K, Henson R, Martin A (2006) Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci 10:14 –23.
Heggelund P, Albus K (1978) Response variability and orientation discrimination of single cells in striate cortex of cat. Exp Brain Res 32:197–211.
Hubel DH, Wiesel TN (1962) Receptive fields, binocular interaction and
functional architecture in the cat’s visual cortex. J Physiol 160:106 –154.
Imig TJ, Adrian HO (1977) Binaural columns in primary field (A1) of cat
auditory cortex. Brain Res 138:241–257.
Kamitani Y, Tong F (2005) Decoding the visual and subjective contents of
the human brain. Nat Neurosci 8:679 – 685.
Kamitani Y, Tong F (2006) Decoding seen and attended motion directions
from activity in the human visual cortex. Curr Biol 16:1096 –1102.
Mehring C, Rickert J, Vaadia E, Cardoso de Oliveira S, Aertsen A, Rotter S
(2003) Inference of hand movements from local field potentials in monkey motor cortex. Nat Neurosci 6:1253–1254.
J. Neurosci., June 30, 2010 • 30(26):8897– 8905 • 8905
Op de Beeck HP (2010) Against hyperacuity in brain reading: spatial smoothing
does not hurt multivariate fMRI analyses? Neuroimage 49:1943–1948.
Pelli DG (1997) The Video Toolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10:437– 442.
Powell TP, Mountcastle VB (1959) Some aspects of the functional organization of the cortex of the postcentral gyrus of the monkey: a correlation
of findings obtained in a single unit analysis with cytoarchitecture. Bull
Johns Hopkins Hosp 105:133–162.
Rasch M, Logothetis NK, Kreiman G (2009) From neurons to circuits: linear estimation of local field potentials. J Neurosci 29:13785–13796.
Rickert J, Cardoso de Oliveira S, Vaadia E, Aertsen A, Rotter S, Mehring C
(2005) Encoding of movement direction in different frequency ranges
of motor cortical local field potentials. J Neurosci 25:8815– 8824.
Sawamura H, Orban GA, Vogels R (2006) Selectivity of neuronal adaptation
does not match response selectivity: a single-cell study of the fMRI adaptation paradigm. Neuron 49:307–318.
Stark E, Drori R, Abeles M (2009) Motor cortical activity related to movement
kinematics exhibits local spatial organization. Cortex 45:418 – 431.
Truccolo W, Friehs GM, Donoghue JP, Hochberg LR (2008) Primary motor
cortex tuning to intended movement kinematics in humans with tetraplegia. J Neurosci 28:1163–1178.
Results II:
The Representation of Visual and Motor Aspects of Reaching
Movements in the Motor Cortex
36
The Journal of Neuroscience, August 24, 2011 • 31(34):12377–12384 • 12377
Behavioral/Systems/Cognitive
The Representation of Visual and Motor Aspects of Reaching
Movements in the Human Motor Cortex
Michal Eisenberg,1,2 Lior Shmuelof,1* Eilon Vaadia,2,3,4 and Ehud Zohary1,2,4
1
Neurobiology Department, Life Sciences Institute and the 2Interdisciplinary Center for Neural Computation, Hebrew University, Jerusalem 91904, Israel,
Department of Medical Neurobiology, The Institute for Medical Research Israel–Canada, Hebrew University, Jerusalem, 91120, Israel, and 4the Edmond
and Lily Safra Center for Brain Sciences, Hebrew University, Jerusalem, 91904, Israel
3
The human primary motor cortex (M1) is robustly activated during visually guided hand movements. M1 multivoxel patterns of functional MRI activation are more correlated during repeated hand movements to the same targets than to greatly differing ones, and
therefore potentially contain information about movement direction. It is unclear, however, whether direction specificity is due to the
motor command, as implicitly assumed, or to the visual aspects of the task, such as the target location and the direction of the cursor’s
trajectory. To disambiguate the visual and motor components, different visual-to-motor transformations were applied during an fMRI
scan, in which participants made visually guided hand movements in various directions. The first run was the “baseline” (i.e., visual and
motor mappings were matched); in the second run (“rotation”), the cursor movement was rotated by 45° with respect to the joystick
movement. As expected, positive correlations were seen between the M1 multivoxel patterns evoked by the baseline run and by the
rotation run, when the two movements were matched in their movement direction but the visual aspects differed. Importantly, similar
correlations were observed when the visual elements were matched but the direction of hand movement differed. This indicates that M1
is sensitive to both motor and visual components of the task. However, repeated observation of the cursor movement without concurrent
joystick control did not elicit significant activation in M1 or any correlated patterns of activation. Thus, visual aspects of movement are
encoded in M1 only when they are coupled with motor consequences.
Introduction
Which parameters are encoded in the primary motor cortex
(M1)? The initial findings in awake behaving monkeys (Evarts,
1968) suggested that M1 is mainly involved in encoding muscle
force, that is, intrinsic parameters of movement. The seminal
work of Georgopoulos and colleagues (1982) in later years indicated that M1 neurons are often tuned to the direction of limb
movement in space and, therefore, encode extrinsic parameters.
Since then, accumulating evidence has suggested that M1 encodes both intrinsic and extrinsic parameters of movement (Scott
and Kalaska, 1997; Sergio and Kalaska, 1997, 2003; Kakei et al.,
1999), and therefore is likely to be involved in a sensorimotor
transformation. For example, using a visuomotor rotation task,
which introduces a constant angular difference between the motor trajectory of the hand and its viewed trajectory on the screen,
Alexander and colleagues showed that M1 neurons are sensitive
to the target position on the screen (Alexander and Crutcher,
Received Feb. 15, 2011; revised June 21, 2011; accepted June 22, 2011.
Author contributions: M.E., L.S., E.V., and E.Z. designed research; M.E. and L.S. performed research; M.E. analyzed
data; M.E., L.S., and E.Z. wrote the paper.
This research was supported by the Israel Science Foundation Grant 39/09 to E.Z. and the Maydan scholarship
provided to M.E. We thank Eldad Assa for programming the task.
Correspondence should be addressed to either Michal Eisenberg or Ehud Zohary, Neurobiology Department, Life
Sciences Institute and the Interdisciplinary Center for Neural Computation, Hebrew University, Jerusalem 91904,
Israel, E-mail: [email protected] or [email protected].
*L. Shmuel’s present address: Motor Performance Lab, Neurology Department, Columbia University, New York,
NY 10032.
DOI:10.1523/JNEUROSCI.0824-11.2011
Copyright © 2011 the authors 0270-6474/11/3112377-08$15.00/0
1990; Shen and Alexander, 1997a). Furthermore, the sensitivity
of neurons in M1 to sensory stimuli often depends on their functional relevance: when the color of a visual cue carried crucial
information to indicate the required hand movement, a considerable fraction of M1 neurons (20%) displayed color sensitivity
(Zach et al., 2008).
We have previously shown, using event-related functional
magnetic resonance imaging, that the multivoxel pattern of
activation in human M1 conveys information about the direction of hand movement (Eisenberg et al., 2010). In that experiment, participants performed a “center– out” task in which
they had moved a cursor to equidistant targets on the screen,
using a joystick. The resulting multivoxel activation patterns in
M1 were more correlated during similar direction movements
than dissimilar ones. Note, however, that this sensitivity to the
direction of movement may stem from the motor command to
the hand, but it could also be due to the covarying visual (and
attentive) aspects of the task, such as the target location or the
cursor trajectory. To that end, following Shen and Alexander
(1997a), we designed an fMRI experiment that allowed separation of the visual elements of the task from its motor aspects.
First, participants completed a “baseline” center– out run, in
which the visual and motor components matched. Next, they
were introduced with a rotation perturbation, in which the observed cursor trajectory was consistently rotated 45° counterclockwise (CCW) with respect to the hand movement. After
practicing the task for a few minutes, participants completed a
full rotation run while being scanned. We then compared the
12378 • J. Neurosci., August 24, 2011 • 31(34):12377–12384
Eisenberg et al. • Visual and Motor Representations in Motor Cortex
elicited multivoxel pattern activations in M1 during the baseline
run and the rotation run. We found that similar hand movements
elicited positive correlations between the evoked multivoxel patterns, as expected. More important, conditions that shared the
same cursor trajectory and target location but differed in their
hand movements also elicited similar positive correlations. On
the other hand, there was no evidence for sensitivity to cursor
direction in M1 when participants passively viewed all visual aspects of the task (in the absence of joystick control). This suggests
that the visual aspects of hand movement are encoded in M1 only
when they are tightly coupled with motor consequences.
Materials and Methods
Participants. Twenty-three right-handed volunteers with normal or
corrected-to-normal visual acuity and no neurological or psychiatric history (13 women and 10 men, aged 18 –35 years) participated in the present experiments. Five of the participants were excluded from further
analysis for various reasons (see below). The experimental procedure was
approved by the Hadassah Ein Kerem Medical Center Ethics Committee.
Written informed consent was obtained from each participant before the
scan.
MRI acquisition. The BOLD fMRI measurements were performed in a
whole-body 3T TRIO SIEMENS scanner. The fMRI protocols were based
on a multislice gradient echo-planar imaging and a baseline head coil.
The functional data were obtained using the following parameters: TR ⫽
2000 ms, TE ⫽ 30 ms, flip angle ⫽ 90°, imaging matrix ⫽ 64 ⫻ 64, voxel
size ⫽ 2.75 ⫻ 2.75 ⫻ 3.1 mm. The 30 –35 slices (with gap of 0.3 mm) were
oriented in the axial position. The scan covered the whole brain. Each of
the two functional runs comprised 616 volumes and contained 250 trials.
Experimental paradigm. The experimental design is the same as described in our previous study (Eisenberg et al., 2010). Seventeen participants were scanned while carrying out a center– out reaching task. Using
an MRI-compatible joystick, they moved a cursor from the center toward
five different targets located in the periphery. The baseline center– out
reaching task was composed of 250 trials. Next, the participants practiced
for ⬃10 min (inside the scanner) on the visuomotor rotation task, in
which the cursor was rotated by 45° CCW with respect to the hand
movement. Thus, for the cursor to move toward the target, participants
had to move the joystick toward the neighboring (clockwise) target without seeing their hand. The participants had adapted to this new mapping
rule implicitly. After the participants reached the targets successfully,
they were scanned again while carrying out another 250 “rotation” trials
(Fig. 1). Each of the five movement directions (i.e., 0, 45, 90, 135, and
180°) was performed 50 times in each run during the course of two 20
min rapid event-related fMRI scans.
Each trial lasted 4 s. The trial began with a red circle at the center of the
screen (“origin”) (Fig. 1). At this stage, the participants had to hold the
MRI-compatible joystick still and make no hand movement. After an
interval of 500 ms, five circles appeared at the upper half of the screen,
spread around the center at equal distances, between 0° and 180°, at steps
of 45°. Four of the circles were blue and one circle was green, signaling the
required future direction of movement. The participants had to keep
their hand still for 2 s until the red “origin” circle turned into a white
cursor. They were instructed to respond by moving the cursor toward the
green target using the joystick. Participants had 1.5 s to reach the target.
Upon reaching the target, all circles disappeared. This served as a cue for
the participants to release the joystick (by relaxing the spring), which
resulted in the joystick returning to its starting position at the center.
There was no explicit failure signal. If the participants did not reach the
target within the 1.5 s time limit, all circles disappeared and the next trial
began. There were also intermittent null trials (2– 8 s long), in which the
red origin and all five blue circles appeared on the screen (thus no target
was distinctly marked for movement). These trials were obviously not
followed by a “go” signal. During these null trials, the participants were
instructed not to move the joystick until the next trial began.
The order of the movement trials in each run was counterbalanced
(first order) and embedded with null trials of various lengths using
OptSeq software. The different lengths of the null trials were used as
Figure 1. Experimental design. Trial flow (left to right): each trial started with the presentation of a red circle at the center of a screen (“origin”). Then, 0.5 s later, five circles appeared in
the periphery, four blue circles and one green circle, which indicated the future target position.
The red “origin” changed its color to white 2 s later, instructing the participant to move the
cursor (the white circle) toward the target (green circle). The participant had 1.5 s to complete
the movement. In the baseline run (top), the cursor movements matched the joystick direction
(joystick forward led to upward cursor movement, etc.). In the rotation run, however, to bring
the cursor to the target (white line), participants had to move the joystick 45° to the right
(dashed white line).
jittered intertrial intervals to allow a more efficient estimation of activation (Dale, 1999). The total length of all null trials was equal to the total
length of each of the five movement conditions (50 trials ⫻ 4 s each ⫽
200 s). Each run began and ended with 16 s of a null event. The task was
programmed with MATLAB version 7.1 (MathWorks), using Psychtoolbox (Brainard, 1997; Pelli, 1997).
Throughout the session, participants were lying in the supine position
inside the scanner, holding the joystick with their right hand while the
targets and cursor were projected onto a screen in front of them using a
projector (Epson MP 7200). The screen was made visible to the participants through a tilted mirror above their faces. Due to their posture,
participants were not able to see their hand or the joystick during the
experiment. Note that in this setting, participants were moving the joystick in the horizontal plane, and the targets and the cursor were observed
on the screen in the vertical plane. Therefore, the observed cursor movement was in a different location than the actual hand movement, even in
the “baseline” condition. However, learning this mapping is almost immediate, as it is similar to our everyday experience, for example, moving
the cursor on the computer screen using a mouse.
Observation paradigm. We ran a control study on six naive participants, in which they only observed the cursor movements of a center– out
task. The cursor movements toward the target were a recording of earlier
joystick-induced movements made by the experimenter. During this
task, participants were instructed not to move their hands, so that any
BOLD activation would be due to mere observation. Participants were
instructed to covertly rate the accuracy of the cursor movement on each
trial (between 1 and 3, where 3 was most accurate) to require them to pay
attention to the cursor movement while avoiding any motor actions. To
ensure that they performed the task correctly and were indeed paying
attention, two of the participants were scanned for an extra 4 min run, in
which they performed the ranking by pushing buttons of an MRIcompatible response box. The two participants rated the accuracy of
movement almost identically (r ⫽ 0.91, p ⬍ 0.0001), and their ratings
were significantly negatively correlated with the angular error of movement trajectory [the angle between the actual movement direction at
peak velocity and the required one; r (subject 1) ⫽ ⫺0.47, r (subject 2) ⫽
⫺0.48, p ⬍ 0.005]. The session was composed of one observation run
(125 trials), then a baseline run with the joystick (250 trials), followed by
another observation run (125 trials).
MT localizer. Three of the six control participants were scanned during
an MT localizer task. The scan included “stationary” condition blocks, in
which stationary concentric white rings were shown at a low contrast,
Eisenberg et al. • Visual and Motor Representations in Motor Cortex
and “motion” blocks, during which rings were alternately expanding and
contracting (in 4 s cycles). The task began with a 33 s stationary block,
followed by 12 interleaved “motion” and “stationary” blocks, each lasting
12 s. The scan ended with a 15 s stationary block. In total, the length of the
scan was 5:24 min. Participants were instructed to maintain fixation on a
red dot in the center of the screen throughout the scan.
Data analysis. Preprocessing and defining regions of interest (ROIs)
were done using Brain Voyager QX (Brain Innovation). The first two
functional images of each run were discarded to allow for stabilization of
the signal. The images were superimposed on two-dimensional anatomical images and incorporated into the three-dimensional datasets
through trilinear interpolation. Before statistical analysis, head motion
correction and high-pass temporal filtering in the frequency domain
(three cycles/total scan time) were applied to remove drifts and to improve the signal-to-noise ratio. The complete dataset was transformed
into Talairach space and Z-normalized.
Next, we applied the deconvolution method to extract the underlying
BOLD response function (the deconvolution kernel, consisting of 10 
weights) for each condition from the acquired signal. This method is
described in detail in our previous study (Eisenberg et al., 2010). Note
that this method makes no a priori assumptions about the shape of the
hemodynamic response function. This analysis resulted in an estimate of
the kernel response amplitudes for 10 independent time points (2 before
the trial, 2 during the trial, and 6 after each condition).
Regions of interest. Cortical regions involved in the visuomotor
reaching task were individually defined based on joint functional and
anatomical criteria, applied separately in each participant. The functional criterion for each M1 voxel was the presence of significantly
higher BOLD activation during the movement trials (regardless of
direction) than during null trials [p ⬍ 0.01, false discovery rate (FDR)
correction] in the baseline run for all participants (including both test
and control groups) according to deconvolution analysis. Active voxels
were included in the M1 ROI if they were within a spatially contiguous
cluster in the anterior side of the central sulcus of the contralateral (left)
hemisphere.
Voxels putatively belonging to the human medial temporal ROI
(hMT⫹) were defined in the control group based on the “observation”
run (GLM, observed cursor movements in all directions ⬎ rest; p ⬍ 0.01,
FDR correction). The location of hMT⫹ was verified in three of the six
participants by running an independent MT localizer, using a direct
contrast between “movement” and “stationary” conditions (GLM, p ⬍
0.01, FDR correction). The average Talairach coordinates of hMT⫹ ROI
in the observation runs (across three participants) were as follows: X ⫽
42, Y ⫽ 66, Z ⫽ ⫺3. This corresponded well with its location during the
hMT⫹ localizer scan in the same participants: X ⫽ 40, Y ⫽ ⫺67, Z ⫽ ⫺1.
Analysis of spatial patterns of fMRI response. The criteria for inclusion
of participants in further analysis were that they successfully reached the
target in at least 80% of the trials and that the fMRI activation elicited in
M1 was above threshold ( p ⬍ 0.01 after FDR correction) in at least 50
functional voxels. Five of the participants were excluded from further
analysis on these grounds (three failed to reach the target in ⬎20% of the
trials, and two elicited insufficient fMRI activation).
Further analysis was done using MATLAB R2007b (MathWorks). We
applied the deconvolution method on each of the two runs separately and
estimated the hemodynamic response ( weights) for each movement
direction in each voxel separately. The activation level (per direction) was
defined by the mean of the  weights 6 and 8 s after the beginning of the
trials (4 – 6 s after movement initiation, which generally corresponded to
the peak activation). Then, to remove activation differences between
voxels that were unrelated to movement direction, in each dataset and for
each voxel, we centered the extracted activation level for each direction
by subtracting the voxel’s mean activation level (across all directions).
This analysis resulted in two matrices, one for each run. Each matrix
consisted of five columns, one for each direction, with its length equal to
the number of voxels in the ROI. The entries in each row of the matrices
were the five centered activation values of a single voxel for the five
directions of movement.
Next, we calculated the correlation coefficient (CC) between the columns of the baseline and the rotation run (each corresponding to the
J. Neurosci., August 24, 2011 • 31(34):12377–12384 • 12379
pattern of activation across all voxels for a given condition). If the estimated  weights contained information about the direction of movement, we expected to find a higher CC for vectors that represent
movements to the same direction (in the two datasets) compared with
the CC calculated for two different directions. Because the CCs were
between baseline movements and rotated cursor movements, we could
distinguish between conditions that shared the same hand movement
and those with the same visual aspects (i.e., target location and cursor
trajectory).
The CCs between compared movements typically drop with increased
angular difference (Eisenberg et al., 2010). Because the rotation angle was
rather small (45°), the positive CCs between trials of “same” target locations could be caused by the similar (although not identical) hand movements. To control for this possibility, we calculated the correlations
between neighboring movement directions (⫺45°; CW instead of CCW)
in which the visual aspects of the task (target location and cursor trajectories) differed by 90°. These CCs served as a “target control” group.
Similarly (although highly unlikely), a positive CC between same hand
movements could result from the fact that the two movements have
similar target locations (separated by only 45°). The correlations between
neighboring targets (⫺45°) with different hand movements (separated
by 90°) served as “movement control.” To assess the statistical significance of the correlations, we applied the Fisher’s z transformation to the
CCs (thereby converting the CC values into a normally distributed variable amenable for parametric statistical testing) and then used paired t
tests across participants to compare the visual and the motor CCs with
their control CCs. Finally, in the separate “observation” scan, performed
in a smaller control group (n ⫽ 6), the CCs between “observation” runs
for same movements versus different movements were compared using
the nonparametric Wilcoxon rank-sum test.
Analysis of accurate trajectories. Despite some practice that participants
had before the rotation run, trajectories in some rotation trials tended to
be slightly curved. This could possibly lead to presumed visual correlations between a given rotation run and its corresponding baseline run,
which are actually due to hand movement. To minimize these effects, we
repeated the correlation analysis using only the more accurate trials. To
that end, we divided the trials of each direction in the rotation run into
two groups of 25 trials each according to their accuracy. Accuracy was
determined by the angular error of movement trajectory at peak velocity.
Trials were classified as “inaccurate” when the angular error was larger
than the participant’s median. Trials in which the target was not reached
within the time limit were considered to have large angular errors regardless of actual trajectory curvature. The mean cutoff angular error was
11.4°. The baseline run was not divided into large and small errors because movements in this run were relatively straight. The  weights of the
rotation run were estimated using the 25 most accurate movements of
each participant in each direction.
Informativeness of voxels about motor and visual aspects of the reaching
task. Do the visual and motor correlations for similar movements largely
result from the activation patterns of the same set of voxels, or are
there two separate subgroups of voxels, one selective for visual components and the other for motor components? To study this, we
calculated two measures for each voxel based on its sensitivity to the
motor and visual aspects of the task. The motor informativeness (Im)
measure was defined as
冘
冘
N⫽5
Im ⫽
j⫽1
N⫽5
i⫽1
共bsj ⫺ brj 兲2
共bsi ⫺ bri 兲2
,
where bs denotes peak activation during the baseline run, br denotes peak
activation during the rotation run, and i denotes the direction of hand
movement. Thus, bsi ⫺ bri is the difference between activation values
during the baseline run and the rotation run while making the same hand
movement; j denotes direction of the “movement control” (45° CW from
the target direction) such that bsj ⫺ brj is the difference in activation
values for the control condition. Thus, if the direction of hand movement
is the only factor determining the voxel’s fMRI activation, the motor
Eisenberg et al. • Visual and Motor Representations in Motor Cortex
12380 • J. Neurosci., August 24, 2011 • 31(34):12377–12384
informativeness is maximal. Visual informativeness (Iv) was calculated in
the same way:
冘
冘
N⫽5
Iv ⫽
l⫽1
N⫽5
k⫽1
共bsl ⫺ brl 兲2
共bsk ⫺ brk 兲2
,
where k denotes target direction and l denotes the direction of “target
control” (45° CCW from movement direction). Both measures were
used to split the data into the more informative and less informative
halves of the voxel population, in two different ways, according to the
motor and visual criteria, respectively.
Results
Twelve participants were scanned while carrying out a center–
out reaching task in an fMRI experiment. Using an MRIcompatible joystick, they moved a cursor from the center toward
five different targets located in the periphery (between 0° and
180°). The baseline center– out reaching task comprised 250 trials
(50 in each direction). Next, the participants practiced the visuomotor rotation task, in which the cursor was rotated 45° CCW
with respect to the hand movement. After a short practice period,
in which the participants learned to reach the targets successfully,
they were scanned again while performing 250 “rotation” trials
(Fig. 1). The target was reached successfully in 93.6% of the trials
in the baseline run and in 92.9% in the rotation run. The mean
absolute error at peak velocity was 8.4° for baseline runs and 11.7°
for rotation runs.
Within each run, the time course of activation for each voxel
in M1 was analyzed using the deconvolution method, allowing
assessment of the activation profile ( weights) generated by each
of the five directions of movements. For each voxel, the average of
the  weights measured 6 s and 8 s after the beginning of the trial,
which usually corresponded to the peak activation, served as the
parameter of activation level for each direction. This activation
level (per voxel, per direction) was centered by subtracting the
voxel’s mean response across directions.
Multivoxel pattern analysis
We have previously reported that M1 voxels typically display
sensitivity to movement direction (Eisenberg et al., 2010). This
selectivity could arise from the direction of hand movement, but
it could also be due to the covarying visual elements of the task
(i.e., the direction of the cursor trajectory and/or target location).
To distinguish between these possibilities, we calculated the CC
between the multivoxel activation patterns elicited by two movements, one from the “baseline” run and the other from the “rotation” run (Fig. 2a). The resulting correlation matrix is shown in
Figure 2c. Note that in the “rotation” run, the visual attributes
were rotated by 45° CCW compared with the hand movements.
This allowed assessment of the effect of hand movement and
visual attributes separately from one another. Specifically, entries
along the main diagonal of the matrix (Fig. 2c, depicted in blue)
represent the CCs between same target directions that differ in
the directions of hand movement. The entries along the next
diagonal (in green) specify the CCs between movements in the
same direction that differ in their target position and cursor trajectories. In both cases, all entries are positive and the average CC
(across participants and matching conditions) is significantly
greater than zero (motor: t(11) ⫽ 3.34, p ⬍ 0.01; visual: t(11) ⫽ 4.3,
p ⬍ 0.005) (Fig. 2d). This suggests that both motor and visual
directional cues (in the context of the task) are encoded in the
patterns of activation across M1 voxels. However, because the
rotation angle was small (45°), the positive CCs between trials of
“same” target locations may be caused by the similar (although
not identical) hand movements. Indeed, we previously reported
that the patterns of activation for hand movements separated by
45° are still positively correlated (Eisenberg et al., 2010). To control for this possibility, we calculated the correlations between
neighboring movement directions (45° difference) that do not
share the same target location and cursor trajectories (“target
control”, compare with “target alignment” condition; Fig. 2a). A
direct comparison between the CCs evoked by the visual match
alignments (“target”) and the corresponding control alignment
(“target control”) showed that the CC difference between the two
alignments was indeed significant (paired t test; t(11) ⫽ 4.28, p ⬍
0.005). Thus, M1 voxels contain genuine information about the
visual components of the task.
Similarly (although highly unlikely), a positive CC between
same hand movements may result from the fact that the two
movements have similar target locations (separated only by
45°). Not surprisingly, the CC for “movement” alignments
was significantly higher than during “movement control”
alignments (t(11) ⫽ 3.16, p ⬍ 0.01).
The effects of trial accuracy
Another issue of concern is that despite the practice in the “rotation” trials before the scan, participants may not be fully adapted
and, therefore, might show a consistent bias toward the “baseline” movement direction. Indeed, the distribution of errors in
the rotation trials (Fig. 3b) was slightly skewed toward positive
errors (mean angular error ⫾ mean SD across participants ⫽
7.37 ⫾ 13.57°). Although this effect is clearly minor compared
with the 45° angle difference, it could still possibly lead to presumed visual correlations between a given rotation run and its
corresponding baseline run, which are actually due to hand
movement. To minimize these effects, we recalculated the CCs
between baseline and rotation runs, taking into account only the
25 most accurate trials for each direction in the “rotation” run
(now the mean angular error at peak velocity ⫾ mean SD across
participants was 2.78 ⫾ 9.9°). The resulting multivoxel patterns
in M1 still show significantly positive correlations when the rotation run matched the baseline run in both the motor (t(11) ⫽
5.1, p ⬍ 0.0005) and visual (t(11) ⫽ 3.48, p ⬍ 0.01) alignments
(“movement” and “target” comparisons in Fig. 3c, respectively).
These correlations were also significantly greater than their corresponding control alignments (paired t test; motor: t(11) ⫽ 4.22,
p ⬍ 0.005; visual: t(11) ⫽ 3.5, p ⬍ 0.005).
The sensitivity to visual aspects in M1
Having found that M1 voxels contain information about the visual aspects of the task, we were intrigued whether similar encoding of visual aspects would also occur in the absence of any hand
movement, and whether the participants learn to associate the
cursor movements with specific hand movements through prior
visuomotor experience. To that end, we conducted a control experiment on six new participants, in which they passively observed the visual display of prerecorded cursor trajectories
toward the target without making any hand movements. To ensure that participants paid attention to these cursor trajectories,
they were instructed to judge the accuracy of the cursor movements. The session was composed of one “naive” observation run
(125 trials, before the cursor–joystick associations were made),
then a baseline run, during which participants used the joystick to
move the cursor toward the targets (250 trials), followed by another (“postvisuomotor”) observation run (125 trials).
Eisenberg et al. • Visual and Motor Representations in Motor Cortex
J. Neurosci., August 24, 2011 • 31(34):12377–12384 • 12381
Figure 2. Multivoxel spatial patterns of activation contain information about target (and cursor) direction as well as hand movement direction. a, An example showing the procedure of alignment
of movements in the baseline run (black arrows denote hand movement and cursor trajectory), with movements during the rotation run (green arrows denote hand movement, blue arrows denote
cursor trajectory, and the gray circles denote target location). When the movements are aligned according to the direction of joystick movement (“movement alignment,” left), the baseline and
rotation run share the motor component (green arrow) but differ in their visual components (target position and cursor direction). Similarly, during “target alignment” (third column), the two
conditions share the same visual components but differ in their motor aspects (by 45°, shaded wedge). In the “movement control” alignment (second column), both visual and motor aspects were
different in the two runs. Note, however, that the target position in the rotation run was displaced by 45° (CW) compared with its position in the baseline run, as in the “movement alignment”
condition (45°, CCW). Similarly, in the “target control” alignment, the movements in the baseline and rotation runs differed by 45°, as in the target alignment condition (compare the wedges caused
by the green arrows). b, Examples of the multivoxel activation patterns (see color code) in M1 (axial plane, Talairach coordinates: z ⫽ 54) during baseline trials (toward 135°; left) and during rotation
trials in which movement was toward 135° (top right; CC between the two patterns was 0.32); the target was toward 135° (middle right; CC ⫽ 0.39), and the target was toward 90° (movement
control; bottom right, CC⫽ ⫺0.25). c, Matrix of mean CCs (across participants) between multivoxel patterns of the “baseline” and “rotation” datasets for all possible direction combinations. The
main diagonal (blue) shows the “target aligned” CC values, the green diagonal shows CCs between “movement aligned” conditions, the values in the purple diagonal are the “movement control”
CCs, and the ones along the red diagonal are the “target control” CCs. d, Mean CCs across participants and across directions for the different conditions (same color coding as in a– c). “Movement
aligned” CCs are significantly larger than the “movement control” CC values ( p ⬍ 0.01) and “target aligned” CCs are significantly greater than “target control” values ( p ⬍ 0.005). Error bars denote
SEM over participants.
We found little evidence for significant M1 activation during
either observation run (both before and after visuomotor training). Consequently, the signal-to-noise ratio of the individual
voxels was very poor. Indeed, during observation runs, the time
course of only 6% of the voxels in M1 was significantly correlated
with the estimated time course (based on the extracted deconvolution kernels), compared with 89% of the voxels when the same
procedure was applied during the baseline movement runs. Not
surprisingly, therefore, the correlations between the observation
runs and the corresponding baseline runs (when the matched
conditions shared the same direction) were not different from
zero according to a Wilcoxon rank-sum test (W(5) ⫽ 32, z ⫽
⫺1.12, p ⫽ 0.31), and neither were the CCs between the two
observation runs (W(5) ⫽ 36, z ⫽ ⫺0.48, p ⫽ 0.7) (Fig. 4b, solid
line). In contrast, when the baseline run was divided into two
datasets, CCs elicited by same-direction movements were significantly higher than during different-direction movements
(W(5) ⫽ 21, z ⫽ ⫺2.88, p ⫽ 0.002; Fig. 4b, dashed line). In visual
motion sensitive hMT⫹, the voxels’ BOLD activation in the two
“observation” runs was statistically significant (Fig. 4c), and the
patterns of multivoxel activations were significantly more correlated when the observed cursor trajectories were in the same direction than when they were in different directions (W(5) ⫽ 21,
z ⫽ ⫺2.88, p ⫽ 0.002; Fig. 4d). Together, these results indicate
that the lack of correlation between observation runs in M1 was
not due to inattention or lack of vigilance (for further information,
see Materials and Methods section Observation paradigm). The
mere presence of specific visual attributes (i.e., target location and
cursor trajectory) is therefore not enough to generate the visualbased correlations between baseline and rotation runs. The visual
attributes seem to be represented in M1 only when they are coupled
to the movement itself. This may reflect aspects that are inherent to
the visuomotor task (such as movement planning or online visual
feedback) that are obviously essential during both baseline and rotation conditions but are absent in the observation task.
Voxel sensitivity measures for the visual and movement
aspects are independent
Are individual voxels that carry information about the direction
of movement more informative about the visual aspects as well? If
Eisenberg et al. • Visual and Motor Representations in Motor Cortex
12382 • J. Neurosci., August 24, 2011 • 31(34):12377–12384
Figure 3. Accurate and inaccurate movement trajectories. a, Example of cursor trajectories
for the “baseline” (top) and “rotation” (bottom) conditions from one participant in all five
directions. Gray trajectories denote trials that were labeled “inaccurate movements” (according
to their angular error at peak velocity). Failed trials were excluded. b, Histogram of the angular
errors made during rotation trials (collapsed across all directions and across all participants).
Gray columns denote the angular errors of “inaccurate” trials. c, Mean CCs, computed for accurate trials only, across participants and across directions for the different conditions. Movement
CCs are significantly larger than movement control values ( p ⬍ 0.005). “Target aligned” CCs
are still significantly higher than the “target control” values ( p ⬍ 0.005). Error bars denote SEM
across participants.
Figure 5. Motor and visually sensitive voxel populations within M1 are independent. The more
selective voxels for movement and for the visual aspects of the task were independently chosen (see
MaterialsandMethods).Theresultingcorrelationcoefficients(acrossallparticipants)between“baseline” and “rotation” sessions, using the motor-selective voxels (left panel) or the visually selective
voxels (right panel), are depicted in light gray bars. Dark bars in both panels show the original results
using all M1 voxels (as in Fig. 2d). Error bars denote SEM across participants. Notice the elevated
correlation of the motor selective voxels in the movement-matched condition (“movement”; light
gray), compared with the original results (“movement”; dark bar). Importantly, the correlation in the
visually matched conditions (“target”; left panel) does not change significantly beyond its original
value (compare “target” light and dark bars). Similarly, a higher correlation is seen during the visually
matched trials (“target”) in the visually selective voxels (right panel), without a concomitant drop in
the correlation during the movement matched trials (“movement”). The two voxel populations,
therefore, do not entirely overlap, nor are they mutually exclusive.
so, this would suggest that M1 neurons may be sensitive to both
aspects, or at least that the modality-exclusive neuronal populations that are sensitive to visual aspects are in close proximity to
populations sensitive to movement (i.e., within a voxel). To that
end, we selected half of the voxels that were more informative
about movement direction and tested the degree to which their
pattern of activation can convey information about the visual
properties of the trial. The results are shown in Figure 5 (left
panel). As expected, selection of the highly informative “motor”
voxels increased the motor CCs (from 0.26 to 0.41; t(11) ⫽ 2.69,
p ⬍ 0.0005), but importantly, it did not improve the visual CCs
(0.21 vs 0.15; t(11) ⫽ 1.58, p ⬎ 0.1). Similarly, we selected the
visually informative voxels and tested their sensitivity to the direction of hand movement (Fig. 5, right panel). Again, selection
of voxels with a high measure of visual informativeness increased
the visual CCs significantly (from 0.21 to 0.37; t(11) ⫽ 5.5, p ⬍
0.05), but it did not affect motor CCs (0.26 vs 0.25; t(11) ⫽ 0.6, p ⬎
0.5). Thus, whereas visual and motor directional aspects of visually guided reaching movements are represented in the multivoxel patterns of human M1, the visual and motor
representations seem to be independent from one another.
Discussion
Figure 4. The multivoxel patterns of activation during observation trials toward the same directionarecorrelatedinhMT⫹,butnotinM1.a,Exampleofonevoxel’stemporalcourseofactivation(
values) for the five directions of hand movement in M1 (shown in dark arrows). The dashed lines
denote “baseline” movement activation levels, and the solid lines denote activation elicited during
“observation” trials. Black and gray colors denote the first and second dataset, respectively. Note the
characteristic robust fMRI activation during movement trials that is totally lacking in the observation
trials.b,TheCCsbetweenthepatternsofactivationinM1elicitedduringthefirstandseconddatasets.
ThedashedlinedescribestheCCasafunctionoftheangulardifferenceinthedirectionsofmovement,
showing clear direction selectivity. In contrast, during the observation trials, the CCs are close to zero
and independent of the angular difference between the directions of cursor trajectory (solid line). c,
Example of a typical hMT⫹ voxel’s activation time course ( values) for the five directions (denoted
by dark arrows) during the first (black) and second (gray) observation runs. Note the robust fMRI
response for the visual aspects of the task that are totally lacking in M1. d, The CCs between the first
and second observation runs as a function of angular difference between the directions of cursor
trajectory in hMT⫹. Unlike in M1 (solid line in b), we see clear direction tuning.
We found that the multivoxel patterns of activation in M1 during
visually guided reaching movements carry information about
both the direction of hand movement (as implicitly assumed;
Eisenberg et al., 2010), as well as the visual elements of reaching.
These findings are consistent with previous studies of single neurons in monkeys (Alexander and Crutcher, 1990; Lurito et al.,
1991; Shen and Alexander, 1997a). The relevant visual aspects,
however, are represented in M1 only when directly coupled with
movement and do not play a role when the same viewed actions
are passively seen (e.g., in the visual playback “observation” task).
A recent fMRI study (Dinstein et al., 2008) has demonstrated
that multivoxel patterns of activation in M1 (during a rock–
paper–scissors game) contain information about the executed
hand configuration. The same voxels, however, do not contain
information about observed hand configurations. This is also
Eisenberg et al. • Visual and Motor Representations in Motor Cortex
consistent with another study in M1 (Chouinard and Goodale,
2009), which found fMRI adaptation (repetition suppression) to
movement but not to the features of the cue that were irrelevant
to the required response.
However, our results are seemingly in contradiction with a
couple of electrophysiological studies in monkeys: Merchant et
al. (2001), who reported that M1 neurons modulated their activity in response to optic flow stimuli in different directions, and
Suminski et al. (2009), who found that visual playback of reaching actions is enough to elicit directional tuning in M1 neurons. It
is likely that the fMRI signal may be too crude to detect neuronal
features that are seen in a minority of the neuronal population.
Ruling out alternative explanations
Humans tend to make eye movements toward the target during
visually guided reaching movements (Gielen et al., 1984; Abrams
et al., 1990; Ariff et al., 2002). Thus, the “visual” sensitivity of the
multivoxel patterns to the target location may actually reflect an
oculomotor sensitivity to the target-specific eye movements and
not the target location itself. This is highly unlikely because the
oculomotor regions are distinctly separate from the hand-related
regions in M1: it is generally accepted that the primary areas of
representation of eye movements are the frontal eye fields (Chen
and Wise, 1995; Passingham, 1995). Indeed, the single neurons in
monkey M1 that are sensitive to reaching direction (Mushiake et
al., 1997) are not modulated by changes in gaze direction. Similarly, regions in human M1 whose BOLD activation is modulated
by pointing are not sensitive to the direction of gaze (DeSouza et
al., 2000). Still, to rule out this alternative, we analyzed data from
a recent fMRI study by Pertzov et al. (2011), in which participants
made saccades in various directions toward remembered targets.
We found no significant saccade-induced activation or positive
CCs for same-saccade directions in the hand-specific M1 (i.e., the
current study ROI) in a participant who took part in both studies.
We conclude that target-specific eye movements are unlikely to
explain the visual selectivity of the multivoxel patterns to the
target location.
What are the relevant visual features in the display
driving M1?
The visual aspects that affect the pattern of activation across M1
voxels could be related either to target location or to the cursor
trajectory. There is some evidence that neurons in monkey M1
are sensitive to the location (or other features) of the visual cue
(Zhang et al., 1997; Zach et al., 2008; Saleh et al., 2010). However,
previous fMRI studies in humans (Toni et al., 1999; Stark and
Zohary, 2008; Chouinard and Goodale, 2009), which contained a
visual target or a visual cue, did not show any visually induced M1
activation. Unlike these previous studies, our reaching task required continuous visual feedback in addition to the registration
of target location. Perhaps the cursor feedback is more relevant
than the target location to the visual representation within M1.
Another explanation for this discrepancy could be that we used
multivoxel pattern analysis, which can detect differences in representation that other methods (used in the past studies) that
measure the mean activation or adaptation over all M1 voxels
may miss.
Alternative interpretations
We stress that the online visual feedback and the cue are likely to
affect the activation patterns in M1. However, another possibility
is that because the baseline mapping is more intuitive than the
rotation mapping, learning the rotated mapping does not totally
J. Neurosci., August 24, 2011 • 31(34):12377–12384 • 12383
abolish the baseline mapping but, rather, a novel mapping is
established in parallel to the baseline mapping and overrides the
existing default representation. If this is indeed the case, the activation elicited during rotation trials represents not only the newly
acquired mapping but also the baseline (unperturbed) mapping.
Thus, the visual correlations we observed may in fact stem from a
subthreshold (unexecuted) retained motor program that is activated when the cue is presented. There is some evidence that M1
is in fact involved in the retention of the previous motor program.
Activation of M1 using transcranial direct current stimulation
(Galea et al., 2010) during rotation learning led to a slower rate of
forgetting of the newly learned visuomotor transformation. Conversely, disruption of M1 activity using transcranial magnetic
stimulation resulted in a faster rate of forgetting of the newly
learned transformation (Hadipour-Niktarash et al., 2007). In any
case, even if the visually induced correlations are in fact a result of
a remnant motor program, it is important to stress that this program is activated by a visual cue, which is associated with a motor
action.
The motor correlations seen in M1 may also stem from the
proprioceptive reafferent signals rather than the afferent motor
signals. Indeed, Rossetti et al. (1995) have shown that displacement of one’s viewed finger location by the use of prisms biases
pointing to visual targets by only a third of the true prismatic
shift. This suggests that proprioception plays a role in planning
movements as well as vision. In addition, Suminski et al. (2010)
have shown that adding proprioceptive information (about the
hand position), congruent with the visual information, improves
the control of a cursor driven by activity of M1 neurons (in a
brain–machine interface). Because we do not dissociate motor
and proprioceptive components of the task, we cannot rule out
the possibility that the proprioceptive signal contributes, at least
in part, to the motor correlations in M1.
The sensitivity of other motor cortical areas
Previous studies in monkeys (Shen and Alexander, 1997b; Cisek
et al., 2003) have shown that neurons in dorsal premotor cortex
(PMd) were more sensitive to the visual aspects of the task than
M1. We found no evidence for this is our study. There were no
significant visual correlations in PMd (motor correlations were
also much lower). One reason for this discrepancy may be that in
our study M1 voxels displayed a much stronger BOLD activation
than the other motor areas. The poor signal-to-noise ratio in
other areas would clearly mask any possibility of observing strong
motor or visual correlations in secondary motor areas. Therefore,
the level of visual correlations does not necessarily reflect accurately the amount of “visual” neurons in each area. Indeed, Dinstein et al. (2008) found, using a similar multivoxel pattern
analysis in a different motor task, that both motor and visual
representations of the action can be found in the anterior intraparietal sulcus (IPS). Interestingly, as in our study, there seemed
to be little correspondence between the two representations in
anterior IPS: decoding level of the executed movements after
training the classifier to distinguish between the observed movements was indistinguishable from chance.
Summary and future outlook
We have shown that both movement direction and visual aspects
of the task are independently represented in regions encoding
hand movements within M1.
It would be interesting to know whether this reflects a change
in the single unit preference as the trial unfolds. Lurito et al.
(1991) showed that at the population level, M1 neurons tend to
12384 • J. Neurosci., August 24, 2011 • 31(34):12377–12384
change their tuning throughout the trial such that initially they
are sensitive to the target location, and later they become sensitive
to the direction of hand movement, as has been shown by Shen
and Alexander (1997a). With the current temporal resolution of
fMRI, we could not detect this temporal drift. This remains to be
shown by separating different epochs of the trials, using paradigms that allow separation of movement planning from movement execution.
References
Abrams RA, Meyer DE, Kornblum S (1990) Eye-hand coordination: oculomotor control in rapid aimed limb movements. J Exp Psychol 16:
248 –267.
Alexander GE, Crutcher MD (1990) Neural representations of the target
(goal) of visually guided arm movements in three motor areas of the
monkey. J Neurophysiol 64:164 –178.
Ariff G, Donchin O, Nanayakkara T, Shadmehr R (2002) A real-time state
predictor in motor control: study of saccadic eye movements during unseen reaching movements. J Neurosci 22:7721–7729.
Brainard DH (1997) The psychophysics toolbox. Spatial Vis 10:433– 436.
Chen LL, Wise SP (1995) Supplementary eye field contrasted with the frontal eye field during acquisition of conditional oculomotor associations.
J Neurophysiol 73:1122–1134.
Chouinard PA, Goodale MA (2009) fMRI adaptation during performance
of learned arbitrary visuomotor conditional associations. Neuroimage
48:696 –706.
Cisek P, Crammond DJ, Kalaska JF (2003) Neural activity in primary motor
and dorsal premotor cortex in reaching tasks with the contralateral versus
ipsilateral arm. J Neurophysiol 89:922–942.
Dale AM (1999) Optimal experimental design for event-related fMRI. Hum
Brain Mapp 8:109 –114.
DeSouza JF, Dukelow SP, Gati JS, Menon RS, Andersen RA, Vilis T (2000)
Eye position signal modulates a human parietal pointing region during
memory-guided movements. J Neurosci 20:5835–5840.
Dinstein I, Gardner JL, Jazayeri M, Heeger DJ (2008) Executed and observed movements have different distributed representations in human
aIPS. J Neurosci 28:11231–11239.
Eisenberg M, Shmuelof L, Vaadia E, Zohary E (2010) Functional organization of human motor cortex: directional selectivity for movement. J Neurosci 30:8897– 8905.
Evarts EV (1968) Relation of pyramidal tract activity to force exerted during
voluntary movement. J Neurophysiol 31:14 –27.
Galea JM, Vazquez A, Pasricha N, Orban de Xivry JJ, Celnik P (2010)
Dissociating the roles of the cerebellum and motor cortex during
adaptive learning: the motor cortex retains what the cerebellum learns.
Cereb Cortex. Advance online publication. Retrieved July 7, 2011.
doi:10.1093/cercor/bhq246.
Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT (1982) On the relations between the direction of two-dimensional arm movements and cell
discharge in primate motor cortex. J Neurosci 2:1527–1537.
Gielen CC, van den Heuvel PJ, van Gisbergen JA (1984) Coordination of
fast eye and arm movements in a tracking task. Exp Brain Res 56:154 –161.
Hadipour-Niktarash A, Lee CK, Desmond JE, Shadmehr R (2007) Impairment of retention but not acquisition of a visuomotor skill through time-
Eisenberg et al. • Visual and Motor Representations in Motor Cortex
dependent disruption of primary motor cortex. J Neurosci 27:
13413–13419.
Kakei S, Hoffman DS, Strick PL (1999) Muscle and movement representations in the primary motor cortex. Science 285:2136 –2139.
Lurito JT, Georgakopoulos T, Georgopoulos AP (1991) Cognitive spatial–
motor processes. Exp Brain Res 87:562–580.
Merchant H, Battaglia-Mayer A, Georgopoulos AP (2001) Effects of optic
flow in motor cortex and area 7a. J Neurophysiol 86:1937–1954.
Mushiake H, Tanatsugu Y, Tanji J (1997) Neuronal activity in the ventral
part of premotor cortex during target-reach movement is modulated by
direction of gaze. J Neurophysiol 78:567–571.
Passingham RE (1995) The frontal lobes and voluntary action: New York:
Oxford UP.
Pelli DG (1997) The VideoToolbox software for visual psychophysics:
transforming numbers into movies. Spatial Vis 10:437– 442.
Pertzov Y, Avidan G, Zohary E (2011) Multiple reference frames for saccadic planning in the human parietal cortex. J Neurosci 31:1059 –1068.
Rossetti Y, Desmurget M, Prablanc C (1995) Vectorial coding of movement:
vision, proprioception, or both? J Neurophysiol 74:457– 463.
Saleh M, Reimer J, Penn R, Ojakangas CL, Hatsopoulos NG (2010) Fast and
slow oscillations in human primary motor cortex predict oncoming behaviorally relevant cues. Neuron 65:461– 471.
Scott SH, Kalaska JF (1997) Reaching movements with similar hand paths
but different arm orientations. 1. Activity of individual cells in motor
cortex. J Neurophysiol 77:826 – 852.
Sergio LE, Kalaska JF (1997) Systematic changes in directional tuning of
motor cortex cell activity with hand location in the workspace during
generation of static isometric forces in constant spatial directions. J Neurophysiol 78:1170 –1174.
Sergio LE, Kalaska JF (2003) Systematic changes in motor cortex cell activity
with arm posture during directional isometric force generation. J Neurophysiol 89:212–228.
Shen L, Alexander GE (1997a) Neural correlates of a spatial sensory-tomotor transformation in primary motor cortex. J Neurophysiol 77:
1171–1194.
Shen L, Alexander GE (1997b) Preferential representation of instructed target location versus limb trajectory in dorsal premotor area. J Neurophysiol 77:1195–1212.
Stark A, Zohary E (2008) Parietal mapping of visuomotor transformations
during human tool grasping. Cereb Cortex 18:2358 –2368.
Suminski AJ, Tkach DC, Hatsopoulos NG (2009) Exploiting multiple sensory modalities in brain–machine interfaces. Neural Netw 22:1224 –1234.
Suminski AJ, Tkach DC, Fagg AH, Hatsopoulos NG (2010) Incorporating
feedback from multiple sensory modalities enhances brain–machine interface control. J Neurosci 30:16777–16787.
Toni I, Schluter ND, Josephs O, Friston K, Passingham RE (1999) Signal-,
set- and movement-related activity in the human brain: an event-related
fMRI study. Cereb Cortex 9:35– 49.
Zach N, Inbar D, Grinvald Y, Bergman H, Vaadia E (2008) Emergence of
novel representations in primary motor cortex and premotor neurons
during associative learning. J Neurosci 28:9545–9556.
Zhang J, Riehle A, Requin J, Kornblum S (1997) Dynamics of single neuron
activity in monkey primary motor cortex related to sensorimotor transformation. J Neurosci 17:2227–2246.
Results III:
Repetition Suppression in the Primary Motor Cortex
The findings presented in this chapter have yet to be published in the scientific
literature
45
Abstract
Repetition suppression (RS) is the reduction of the functional MRI response to a repeated
stimulus. This phenomenon has been shown for various kinds of visual stimuli (e.g. faces,
objects, etc) in many areas along the visual pathway. Recently, RS has been shown in motor
areas as well, when repeating a movement to the same direction as in the previous movement.
The interest in RS has been enormous because it was suggested to reflect the tuning properties of
single neurons, thereby allowing an indirect study of the properties of neuronal populations,
using a noninvasive technique. Several models have been suggested to explain the phenomenon
at the neuronal level. To that end, elecrophysiological studies designed to test which of these
alternative models best explains RS have been carried out in monkeys. The findings vary
between different visual areas. In the inferior temporal area (IT) for example, RS was found
mostly for the preferred stimulus, consistent with the interpretation of synaptic fatigue, whereas
the pattern of RS in middle temporal cortex (MT) fit the model of sharpening of the tuning curve.
In motor areas this issue has not yet been studied. In this study, we analyze electrophysiological
data from macaque primary motor cortex, during center-out reaching movements in eight
different directions. We utilize the task design, in which the same movements were sometimes
repeated in successive trials while at other times successive movements were in opposite
directions, to look for a difference in the firing rate and/or in the local field potential (LFP)
between the same current movements, depending on the movement history (i.e., same or
opposite direction of movement in the previous trial).
Our goal here was to look for an adaptation mechanism, possibly similar to one of the
previously suggested mechanisms, to shed light on the neuronal basis for RS in the motor
pathways. However, unlike the studies in the visual system, we found no sign of RS in the single
unit database or in the LFP. Further study is required to fully understand the discrepancy
between these neuronal results and the RS found in the fMRI signal, but the lack of any neuronal
repetition effect, could suggest that the RS found in the fMRI signal of the motor cortex does not
reflect a neuronal process as generally accepted.
46
Introduction
The functional MRI (fMRI) response to a repeated stimulus is often reduced compared to the
response to a non-repeated stimulus. This phenomenon is known as repetition suppression (RS),
and has been shown for many different visual characteristics, from intensity and contrast in the
early visual cortex (Gardner et al., 2005) to objects in higher areas, such as the lateral occipital
complex (Buckner et al., 1998; Grill-Spector et al., 1999; Kourtzi and Kanwisher, 2001).
The importance of fMRI RS derives from suggestions that it can be used as a tool to assess
neuronal selectivity (Tootell et al., 1998; Grill-Spector and Malach, 2001; Avidan et al., 2002).
Several models have been proposed to explain the fMRI RS at the level of single unit activity:
(1) fatigue. According to this model all neurons show a proportionally equivalent decrease in
activation in response to a repeated stimulus. The neural mechanism in this case could be either
firing rate adaptation or synaptic depression (short term or long term). (2) Sharpening. According
to this model we expect a decrease in activation only in neurons that are less responsive to the
presented stimulus. Neurons that respond optimally to the stimulus will show no change in
activity in response to repetition (3) facilitation. This model predicts that repetition will facilitate
faster processing of the stimulus, and therefore a shorter duration of the neuronal firing (GrillSpector et al., 2006; See figure 1).
47
Figure 1: Neural network models for RS. (a) The visual stimulus is assumed to cause
activity in the input layer (corresponding to early visual cortex) before being processed
in a hierarchical sequence of stages. The blue graphs indicate spiking (as a function of
time) of the neurons with highest response at each stage (indicated by black circles). (b)
Because the BOLD signal integrates neuronal activity over time, all three of these
models predict reduced BOLD for repeated stimuli, but for different reasons: Fatigue
model (left, lower firing rates); Sharpening model (centre, fewer neurons responding);
Facilitation model (right, shorter duration of neural processing). Grill-Spector et al.,
2006.
A decrease in the firing rate of single units has been shown for non-human primates in response
to repeated stimuli in the primary visual cortex (V1) (Movshon and Lennie, 1979; Müller et al.,
1999; Sharpee et al., 2006), in the inferior temporal cortex (IT) (Sawamura et al., 2006; De
Baene and Vogels, 2010) and in the middle temporal cortex (MT) (Kohn and Movshon, 2004). In
IT (De Baene and Vogels, 2010), as well as in V1 (Movshon and Lennie, 1979; Müller et al.,
1999), firing rate decreases most when the adapting and test stimuli are the same, concurring
with the fatigue model, while in MT, Kohn and Movshon (2004) have shown a decrease in firing
rate in stimuli near the adapter but not in the exact same stimulus, which concurs with the
sharpening model.
48
Recently, fMRI RS has been shown in the primary motor cortex (M1) for movements as well, by
us (Eisenberg et al., 2010) and by others (Dinstein et al., 2007; Fabbri et al., 2010). However, it
has never been studied in single unit activity, and it is unknown whether RS can be found at the
neuronal level in motor areas or if it occurs in a pattern similar to those found in visual areas.
In this study, I analyzed electrophysiological data recorded from macaque M1, and tried to find a
difference in responses to repeated movements compared to non-repeated movements. I utilized
the task design, in which movements in the same direction were sometimes repeated in
successive trials, while at other times successive movements were in opposite directions. My
goal was to determine if RS can be found in the single unit activity of M1, and test whether the
change in response fits any of the suggested models explaining RS of the fMRI signal. In
addition, I compared the local field potential (LFP) in M1 that was generated by repeated and
non-repeated movements. The LFP is generally considered a population signal of many
thousands of neurons, reflecting mainly the synaptic inputs (Mitzdorf, 1987). It has been found
to correlate with the BOLD signal better than single- and multi-unit firing rates (Logothetis et al.,
2001). Therefore, it seemed feasible that the RS patterns seen at this level may be more
correlated with the BOLD signal. The results of the experiments discussed in this section show
no difference between repeated and opposite movements, either in the single unit data or in the
LFP signal. This could suggest that the RS found in the fMRI signal of the motor cortex does not
reflect a neuronal process, as generally accepted.
Materials and Methods
Animals, recordings and behavioral task
One male monkey (Macaca fascicularis, 4 kg) was chronically implanted with a
microelectrode array (Cyberkinetics Neurotechnology Systems) under anesthesia and aseptic
conditions, in the arm region of M1, contralateral to the performing arm. Animal care and
surgical procedures complied with the National Institutes of Health Guide for the Care and
Use of Laboratory Animals and with guidelines defined by the Institutional Committee for
Animal Care and Use at the Hebrew University.
49
Monkeys used a robotic arm (Phantom Premium 1.5 High Force; SensAble Devices) to control
the movements of a cursor on a video screen in a two-dimensional plane. Before surgery,
monkeys were well trained to perform a standard eight-target center-out reaching task using this
device. The robotic arm moved the cursor from the starting point at the center of the screen to a
visual target in a delayed go-signal paradigm. Each trial began when the monkey positioned the
cursor at the central circle. After a variable hold period of 0.75–1.25 s, a target appeared at one
of the eight possible positions, which were uniformly distributed in a circle 4cm from the center.
After an additional 0.75–1.25 s hold period, the central circle disappeared (go signal), prompting
the monkey to move to the target in less than 1 s. This generous time constraint allowed
relatively natural reaching movements. After another 0.4s, a liquid reward was delivered. A
scheme of the trial flow is shown in figure 2.
Session flow: Each session began with standard trials (with no perturbation) introduced in sets of
three trials with targets in the same direction in a row, and after completion of the set, another set
was presented with the neighboring clockwise target, etc. After completing trials in all eight
directions, the next round was the same, except the directions changed in a counter-clockwise
order. Finally, the third round had random assignments of the eight movement directions. In the
second part of the experiment (block 2), the monkey was introduced with a visuomotor rotation,
where in order to move the cursor in one direction the monkey needed to move its arm in another
direction. There were several days in which block 2 had no rotation, and the monkey simply had
to make repeated movements to the same specified direction. (In each session the repeated
movement was to a different direction). Block 3 was standard again (with no rotation). In this
block the sequence of target appearances was in a pseudo-random order.
Monkeys were trained for several months with the default eight-target task.
For further analysis we did not use the first standard block, where trial directions did not appear
randomly, or the rotation block. Therefore, we focus here on the third block, which was the
second standard phase. Since the rotated movement in the previous block probably caused an
aftereffect (i.e., movements were curved to the direction opposite from the learned direction), at
least in the first several trials in the learned direction (Paz et al., 2003), we excluded the rotated
direction from the analyses, thereby nullifying the learning affect.
50
a
trial start
target appear
0.75-1.25 s
0.75-1.25 s
go signal
movement
<1 s
b
time
Figure 2: Trial flow. (a) Each trial began with the appearance if the central target
(“origin”). The monkey had to hold the red X (the cursor) inside the origin for a variable
hold period of 0.75-1.25 s. Next, a target appeared in one of 8 possible positions. The
monkey had to keep the cursor in the origin for another 0.75-1.25 s. After this second
hold period, the origin disappeared and the monkey had to move towards the target
within 1 s. In this example there are two consecutive trials in the same direction (45 0).
(b) An example of two consecutive opposite trials, where a trial in the direction of 45 0
came after a trial with a target in 2250.
Neuronal data analysis
Neurons were selected on the basis of their directional tuning. The sample includes neurons with
tuning curves that show a good fit (R2>0.65) to the cosine function [r(d) = a + b * cos(d − d0)]
(Georgopoulos et al., 1982). The reasoning for choosing directionally selective neurons was that
the RS can be explored separately for different distances from the preferred direction (PD),
helping to distinguish between the different models explaining the fMRI RS.
Firing rate was computed for the epochs 100 to 600 ms after target onset and 200 ms before
movement onset to 300 ms after movement onset.
51
Repeated and opposite trials: For the main analysis I used repeated trials, which were sequential
trials with the same movement direction, and opposite trials, which were 1800 from the
movement in the previous trial. The opposite trials were used instead of taking all non-repeated
trials, because some degree of adaptation is expected for movement directions that differ slightly
(e.g. 450) from the previous trials. We wanted to examine the maximal effect by comparing the
most expected amount of adaptation (repeated movements, which were identical to the previous
movement) with the least expected amount of adaptation (opposite directions, which differed the
most from the previous movement, at least in the population of neurons activated). Note that
during performance the monkeys could not anticipate the direction of the next trial, since the
trials were introduced in a pseudo-random fashion. Thus, there was no fixed number of repeated
trials. We discarded sessions with less than two repeated trials or opposite trials in each
direction.
Adaptation Index (AI) was calculated for each neuron, and was defined as
,
where Oi is the mean response of the opposite trials in the ith direction, with i ranging between 1
to 8, and Ri is the mean response of the repeated trials in the ith direction. The AI was computed
for the mean response both after target appearance, and around movement onset.
Tuning Curve: For each neuron the tuning curve was computed by averaging over trials in each
of the 8 directions, and was fitted to a cosine function. We computed the R2 and the PD (the
angle corresponding to the peak of the cosine wave). For the comparison between repeated and
opposite trials at the PD and at the direction opposite from the PD we used the direction of
movement closest to the PD, and the direction farthest from the PD, respectively.
LFP data analysis
For the LFP analysis we averaged the recorded signal in the low frequency band (0.3-250 Hz)
from each electrode over trials in each direction separately, and found the second positive peak
(P2) of the movement evoked potential (mEP). P2 was the first positive peak after the first
negative peak (N1), and was defined as the maximum value in the range of 350 to 1150 ms after
movement onset. This peak was shown to have the highest signal-to-noise (at the time domain)
by Rickert and colleagues (2005).
52
Next, we fitted the tuning curve of the peak-to-peak amplitudes for the various directions to a
cosine function, as was done for the single unit data, and calculated the R2 and the PD.
Paired T-tests were used for the single units and for the LFP data to assess significance in all
comparisons between the repeated trials and the opposite trials.
Results
The monkey made center-out reaching movements towards 8 targets in the periphery, presented
in a pseudo-random fashion. We used this task design to separately analyze trials in which the
same movement direction was repeated in consecutive trials (“repeated”) and trials in which the
current movement followed movement in the opposite direction in the previous trial
(“opposite”).
Performance in repeated and non-repeated movements
First of all I wanted to see if a learning process was still going on, and if there was any difference
between the repeated and non-repeated movements in how straight the movement trajectories
were to the target. To that end I assessed the angular deviation for each trial by calculating the
angle between the actual trajectory at the peak velocity and the direct trajectory to the center of
the target. For non-repeated movements the angular deviation was 7.360, and for repeated
movements it was 6.650. The difference was not significant (t(509)=1.4, p=0.16). The angular
deviations were not significantly different in the fMRI experiment either (t(10)=0.8, p=0.44).
Neuronal data analysis
I analyzed the activity of neurons that were directionally tuned during two time epochs: (a) target
related activity, 100 ms to 600 ms after target appearance; and (b) movement related activity,
53
200 ms before to 300 ms after movement onset. Sixty two neurons (37% of all neurons) were
classified as “target-related” based on their directional selective activity in the interval following
target appearance. One hundred and seventeen neurons (70% of all neurons) had movementrelated activity (i.e., directional selectivity during the movement execution interval).
For each neuron, we plotted the peri-event time histogram (PETH) of each of these two
conditions. We found no difference in the overall firing rate between trials in which the direction
was repeated ("repeat trial") and trials in which the previous movement was in the opposite
direction ("opposite trial"). We quantify this by the adaptation index (AI). We show that this
was true when the PETH was aligned to both the movement onset (figure 3a, t(116)=-1.34,
p=0.18) and to target appearance (figure 4a, t(61)=-0.96, p=0.34). Next, for each neuron we
calculated the AI (see materials and methods). The AI of a neuron should be positive if its firing
rate is lower for repeated trials or negative if its firing rate is higher for repeated trials. If there is
no adaptation, the AI should be 0. In figure 3b we show a histogram of the adaptation indices
across all directionally tuned neurons during movement. We found that the AIs were distributed
around 0 (mean AI=-1.2%; t(116)=-0.69, p=49). Therefore, no adaptation was found. We
calculated the AI after target appearance as well (figure 4b), and here we find no significant
difference
from
either
(mean
AI=1.4%;
b
Number of neurons
Firing rate [spikes/sec]
a
0
Time [ms]
Adaptation Index [%]
54
t(61)=0.8,
p=0.43).
Figure 3: Directionally selective M1 neurons show no RS around movement onset. (a)
PETH of the mean over the entire sample of selected M1 neurons for repeated trials
(blue) and for opposite trials (red) in all directions of movement. Time point 0
indicates movement onset. Gray lines denote the PETHs for opposite trials +/-standard
error of opposite trials. (b) Histogram of AIs for movement related activity. The arrow
denotes the mean AI value. Note that the mean AI is not significantly different from 0,
indicating that there is no significant adaptation.
b
Number of neurons
Firing rate [spikes/sec]
a
Time [ms]
Adaptation Index [%]
Figure 4: Directionally selective M1 neurons show no RS after target appearance. (a)
PETH of the mean over the entire sample of selected M1 neurons for repeated trials
(blue) and for opposite trials (red) in all directions of movement. Time point 0
indicates target appearance. Gray lines denote the PETHs for opposite trials +/standard error of opposite trials. (b) Histogram of AI for target related activity. The
arrow denotes the mean AI value. Note that the mean AI is not significantly different
from 0, indicating that there is no significant adaptation.
Another possibility is that the adaptation might manifest itself by a change in the tuning curve
width. For example, repeated movement would lead to a decrease in activation only in directions
close to the PD of the neuron, leading to a wider tuning curve; or a decrease in firing only in
directions far from the PD, causing a narrowing of the tuning curve. To that end, I computed the
55
direction tuning curve of each neuron, for the repeat and opposite conditions, separately, and
then aligned the resulting tuning curves according to the direction of movement closest to their
PD. Again, we found no significant difference between repeated and opposite directions for any
of the distances from the PD either for the movement-related activity (figure 5, at PD for
example, t(116)=1.36, p=0.18) or for the target-related activity (figure 6, at PD t(61)=0.99, p=0.33).
Figure 5: Directional tuning of repeated vs. opposite trials around movement onset.
(a) PETHs of the mean over the entire sample of selected M1 neurons for repeated and
opposite trials in the PD of each neuron. (b) PETHs for repeated and opposite trials in
the direction 180 degrees from the PD of each neuron. (c) Tuning curves for repeated
(blue) and for opposite (red) trials. Error bars denote standard error of the mean over
neurons.
56
Figure 6: Directional tuning of repeated vs. opposite trials after target appearance. (a)
PETHs for repeated and opposite trials in the PD of each neuron. (b) PETHs of the
mean over the entire sample of selected M1 neurons for repeated and opposite trials in
the direction 180 degrees from the PD of each neuron. (c) Tuning curves for repeated
(blue) and for opposite (red) trials. Error bars denote standard error of the mean over
neurons.
The same analysis was carried out over all neurons, with no selection criteria, figuring that this
would be most similar to the fMRI data, which is affected by the entire population of neurons,
not only the well isolated and directionally tuned ones. For these analyses we used 169 neurons.
No difference was found between the repeated and opposite trials around movement onset (mean
AI=-0.19; t(168)=-0.12, p=0.91), or after target appearance (mean AI=0.26; t(168)=0.14, p=0.89).
LFP data analysis
It’s possible that adaptation will be found in the LFP signal, which has been shown to be much
more correlated to the fMRI signal than single unit activity (Logothetis et al., 2001). Finding RS
57
of the LFP signal would suggest that the discrepancy between single unit activity and the fMRI
signal is not due to the difference in species (human vs. nonhuman primates) or in the different
experiment parameters, but rather in the nature of the signal. LFP reflects the input to the area
more than its output (Mitzdorf, 1987), as may be the case with the BOLD signal. Therefore, if
the RS is a synaptic process, it might be reflected in the fMRI and in the LFP signals, but not
necessarily in the single unit activity.
For the LFP data, I did a similar analysis as for the neuronal data. I computed the tuning curve of
each electrode by taking the measure of the second positive peak (P2) of the mEP. There is no
significant difference in the mEP between repeated and opposite trials (t(734)=-0.88, p=0.38;
figure 7a). When I looked only at the activation at the PD or only at the direction 180 degrees
from the PD, I found no difference between repeated and opposite trials either (at PD: t(96)= 0.88,
p=0.38 and 180 degrees from PD: t(88)=0.48, p=0.64; figure 7b & c, respectively).
mEP
b
a
P2
N1
mEP
mEP
c
Time [ms]
Time [ms]
Figure 7: M1 mEPs show no RS around movement onset. (a) MEP for repeated and
opposite trials. The dashed lines marked N1 and P2 indicate the points in time of the
negative and positive peaks of the mEP. (b) MEP for the PD of each electrode (c)
MEP for the direction 180 degrees from the PD of each electrode.
58
I show LFP results only for movement related activity, because when aligned to target
appearance, the data was too noisy to extract a reasonable signal, and it is not clear which
parameter or what time interval is best for comparison of the repeated and the opposite trials.
Discussion
We found no RS in the firing rate of monkey single neurons in M1 at the population level, nor
have we found RS in the LFP. This is in contrast with the RS found in human M1 in functional
imaging studies (Dinstein et al., 2007; Eisenberg et al., 2010; Fabbri et al., 2010). Furthermore,
electrophysiological recordings in visual cortical areas, such as IT and MT, indicate that both
single neurons and the LFP signal do show RS in response to a repeated visual stimulus (Kohn
and Movshon, 2004; De Baene and Vogels, 2010). There are several possible explanations to our
results:
Learning vs. repeating
Recently, there has been growing debate about whether the RS phenomenon is due to an
automatic process, or to a top-down process, reflecting our expectation (Summerfield et al.,
2008; Pedreira et al., 2010; Larsson and Smith, 2012). It is possible that the RS observed during
movement repetition is in fact due to our expectation, and would be better accounted for by
motor learning.
Paz and colleagues (2003) have shown narrowing of the neurons’ tuning curve in M1 when
monkeys were learning a novel visuomotor rotation. This narrowing was found when the
monkeys made repeated movements to the same target with the novel transformation, but not
when they simply repeated the same movement with standard mapping (i.e., no rotation). The
sharpening of the tuning curve, therefore, reflects the learning process taking place in M1.
Similar narrowing of the tuning curve was found by Kohn and Movshon (2004) in MT in
response to repetition of observed moving gratings. However, while in Kohn and Movshon’s
study the narrowing was caused by a decrease in the activity for neighboring directions while
59
maintaining the level of activation in the PD, the narrowing of the tuning in the study by Paz and
colleagues is obtained by an increase in firing rate at the PD.
Figure 8: Sharpening of Tuning curves of example single neurons. (a)
Tuning curves of MT neurons before (white circles) and after (black
circles) adaptation to the PD of these neurons (adapted from Kohn and
Movshon, 2004). (b) Tuning curves of M1 neurons before (gray line) and
after (black line) learning a rotation in a direction near the PD of these
neurons (adapted from Paz et al., 2003).
Perhaps motor RS differs from visual RS, in that it occurs only during learning, when the task is
not automatic. Since the monkey had months of training on the standard center-out movements,
and no difference was found in task performance between repeated and non-repeated
movements, maybe we shouldn’t expect to find adaptation to repeated movements in the same
direction? The explanation for fMRI RS in human participants could be that they weren’t as
proficient at the task as the monkey (they had only several minutes of practice before the task).
However, no difference in performance was found in the fMRI experiment, and there was no
difference in RS between participants who did better at the task (smaller angular deviations), and
participants who did worse (data not shown).
60
Motor vs. visual adaptation
It is possible that the motor adaptation mechanism is fundamentally different from the visual
one. For the perception of sensory stimuli, adaptation makes sense: we need to be alert to
change, as is seen for luminance and contrast in areas as early as the lateral geniculate nucleus
(Solomon et al., 2004) and the retina (Enroth-Cugell and Shapley, 1973; Smirnakis et al., 1997;
Chander and Chichilnisky, 2001). This requirement is irrelevant for voluntary motor actions.
Perhaps the mechanism is different. One speculative possibility is that the BOLD RS we have
found in M1 is merely a residual of visual adaptation to the different targets. However, RS was
recently found in M1, even when there was no visible target or compatible visual feedback
(Dinstein et al., 2007). In addition, to see if RS might be found in M1 during repeated
observation of the same target, we aligned the neuronal responses to target appearance. No
difference was found in the average neuronal response during repeated and opposite trials. It still
remains to be seen whether there is RS in the LFP signal in the frequency domain [perhaps in the
gamma range, as shown by De Baene and Vogels (2010) for visual stimuli], especially when
aligned to target appearance. This would indicate that the fMRI RS could be caused by visual
inputs from other areas, since both LFP and BOLD activation are thought to reflect inputs rather
than action potentials. This is consistent with our finding of RS in anterior and medial
intraparietal areas, as well as in M1 (data not shown).
Is BOLD RS a reflection of adaptation at the neuronal level?
It’s possible that the BOLD RS is an artifact that does not always reflect the neuronal activity,
both in motor and in visual cortex. According to this hypothesis, the adaptation found in visual
areas for firing rate and for BOLD response are two independent phenomena. This is in line with
the results of Sawamura et al. (2006), who found that macaque IT neurons adapt when presented
with two successive different images to which the neurons are equally responsive, but less than
when the repeated stimuli are identical. Therefore, the neuronal adaptation is more selective to
visual stimuli than the neuronal response, complicateing the interpretation of RS of the fMRI
signal. Perhaps the BOLD RS reflects something other than neuronal or synaptic adaptation. It
has been suggested by Krekelberg and colleagues (2006) that the RS attributed to neuronal
adaptation could in fact be due to nonlinearities in the neurovascular coupling process. For
61
example, maybe the blood flows towards a certain population of neurons in excess. When we
reactivate the same population at approximately the same level as in the previous trial, the level
of oxygen in the area is already saturated, which brings to less blood flow in response to
repeating the same trial, and therefore to a smaller BOLD response.
A word of caution is in place here: Before we can accept any of these interpretations, we should
keep in mind that the experiment that was used for my analysis was different from the one
generally used for visual stimuli; The trials here are individual movements with long inter-trial
intervals, while in the visual studies trials are generally comprised of an adapter and a repeated
or non-repeated test stimulus with a short (300 ms) time interval between them (for example see
Sawamura et al., 2006). In future studies the experiment should be run on monkeys before they
reach a performance ceiling affect, so a possible relation between behavioral performance and a
repetition affect can be examined. Additionally, in order to be sure that the difference isn’t due to
the difference in species or in task parameters, the unit recordings should be done in parallel with
the fMRI response in monkeys, or at least in the same monkeys, as was done by Sawamura and
colleagues (2005) for visual stimuli.
62
Discussion
Summary of results
Functional organization of human motor cortex: directional selectivity for movement
In the first chapter of the results I show, using several methods of analysis, that voxels in human
M1 are tuned to the direction of movement. This leads us to the conclusion that neurons in
human M1 are likely to be organized according to their directional preference; i.e., neighboring
neurons tend to have close preferred directions (PDs). Additionally, I use a model based on the
depth of modulation of the fMRI response to the various directions in order to estimate the
average size of a cluster of neurons with the same PDs. We find that the diameter of such a
cluster is of the order of 85-470 micrometers, which is in the same order of magnitude as
estimated in studies with primates (Amirikian and Georgopoulos, 2003; Stark et al., 2009).
The representation of visual and motor aspects of reaching movement in the human motor
cortex
In the second chapter of the results I show that in addition to the robust movement representation
in human M1, there is also a significant visual representation of the direction of motion. This
representation, however, exists only when the visual stimulus is relevant to the motor task, either
by indicating the target, or by providing cursor feedback during the task. When the task required
observation only, with no hand movement, no activation was found in M1, nor was there any
information in the multi-voxel patterns of activation. This supports the notion that M1 plays a
role in a higher level of movement planning than originally accepted.
Repetition Suppression (RS)
In the last chapter I looked for evidence of suppression of neuronal activity in macaque area M1
when repeating a trial in the same direction. However, no such evidence was found. This is in
marked contrast with the RS found in the homologues areas in humans using fMRI, by us
(chapter 1 of results) and in previous studies (Dinstein et al., 2007). This mismatch raises doubts
about the interpretation of the source of RS in the fMRI signal, which is considered to reflect the
63
properties of single neurons (Grill-Spector and Malach, 2001). Therefore, either the mechanism
of RS in the motor cortex is entirely different from sensory areas, or perhaps the mechanism is
not as directly related to neuronal properties as previously thought. I will elaborate on this further
in the next section.
Human fMRI vs. monkey electrophysiology
Overall, the prime goal of this thesis was to study the representation of movement directions in
M1; from how neurons with different PDs are organized in M1 to the representation of the visual
and motor components of reaching. Another goal was bridging over the gap, both between
different methods of data acquisition, i.e., electrophysiology and fMRI, and between different
species, i.e., human and non-human primates, to try to establish commonalities between these
levels.
In the first two studies, I used multi-voxel pattern analysis. Although there is some evidence
from electrophysiological studies done on macaques towards functional organization and
towards a visual representation in M1, these questions have not been previously addressed in
humans. There are many reasons for the scarcity in human imaging studies of movement;
including lack of room for movement inside the scanner, joysticks and devices recording
movement are usually metal and not MRI-compatible, the difficulty of scanning a person making
hand movements without too much head movement (thus lowering the signal to noise ratio), etc.
Despite these constrains, there have been several fMRI studies of reaching (Schaal et al., 2004;
Diedrichsen et al., 2005; Filimon et al., 2007). Dinstein and colleagues (2008) successfully
decoded different hand configurations in M1. They used the rock-paper-scissors game and found
different patterns of activations for each of the three hand configurations. The strength of my
work is the use of reaching movements in different directions (using a joystick) such that the
dissimilarity between their neural correlates could be quantified. I show that the spatial patterns
of activation elicited by different movements change gradually as the angular difference between
the movements increases. This allows us to draw conclusions about the likely functional
organization of neurons in human M1, given certain assumptions about the response of single
64
neurons to reaching movements in different directions from previous monkey studies
(Georgopoulos et al., 1982).
In the third study I focus on finding evidence for repetition suppression (RS) in macaque M1.
This effect that has previously been shown in fMRI studies in humans, and has become one of
the most widely used techniques to indirectly infer the neuronal properties of the studied area
(Tootell et al., 1998; Grill-Spector and Malach, 2001; Avidan et al., 2002). My motivation was to
establish this phenomenon in the motor cortex, and to examine its neuronal mechanism, which is
unclear at this point in time. However, I did not succeed in finding neuronal evidence for the RS
phenomenon in monkey M1. RS has been shown in electrophysiological recordings from
monkeys in visual areas in response to repeated visual stimuli (e.g., De Baene and Vogels, 2010).
It is possible that designing the task differently, placing trials in pairs (an adapter trial followed
by a repeated or non-repeated test trial) rather than in a semi-random order would change our
results by allowing averaging over a larger number of repeated trials and by better controlling the
time interval before the test trials while allowing a longer rest before adapter trials. However, it
is also possible that the relation between the BOLD signal and single-unit activity is not as
generally assumed.
Is RS a bottom-up or a top-down phenomenon?
The origin of RS has recently been under debate. Does RS reflect an automatic process in the
brain, or does it reflect expectation of the coming trial? Several years ago Summerfield and
colleagues (2008) showed that the RS in the fusiform face area is affected by the likelyhood of
the specific face stimulus to appear. They found much weaker RS when the specific stimulus was
improbable (i.e., unexpected). This contradicts models of automatic processes previously
suggested to explain RS, such as synaptic fatigue or sharpening of the tuning curves, and implies
that the RS phenomenon reflects a top-down perceptual “prediction error”. Their results were
later confirmed by Larsson and Smith (2012), who also found that the expectation effect is
attention dependent. By diverting subjects’ attention, they showed that RS still exists in the
absence of the expectation effect. These results suggest that fMRI RS reflects a combination of
attention-dependent expectation effects and neuronal adaptation.
65
When the effect of expectation was tested on macaque inferior temporal (IT) neurons, no
expectation effect was found (Kaliukhovich and Vogels, 2011). This could be explained by the
effect being exclusive to the FFA. Alternatively, this could strengthen our hypothesis, that the
fMRI RS is essentially different from the adaptation found in single neurons in the visual stream.
If we try to interpret these results in ‘motor’ terms, it is possible that there needs to be a learning
process in order to exhibit RS, in an analogy to the effect reported by Paz and colleagues (2003).
They had monkeys repeat a movement to the same direction under a novel visuomotor rotation
for an entire learning block. In response, they found sharpening of tuning curves in neurons with
PDs close to the learned direction. This sharpening was not found when the monkey merely
repeated the same movement with standard mapping, and no learning was required.
RS in human and in monkey
In a study by Sawamura and colleagues (2005), fMRI RS has been shown to be very similar in
humans and in awake monkeys, in homologous areas lateral occipital complex and IT,
respectively, using the same experimental paradigms. Later, Sawamura et al (2006) recorded
from IT neurons in one of the monkeys from the fMRI experiment (thus ensuring that the singlecell recording was done from the same region that exhibited fMRI RS), and found RS in the
firing rate of single neurons as well. However, the stimulus selectivity of the RS effect was
different from the selectivity of the neurons’ response, contradicting the claim that fMRI RS
directly reflects neuronal properties. In light of these studies, the difference I found between the
human fMRI results and the monkey electrophysiological results was more likely due to the
different methods of data acquisition rather than the different species.
FMRI techniques and their relation to neuronal activity
Many fMRI studies done on humans in the past couple of decades are in agreement with parallel
electrophysiological studies, but in the large majority of fMRI studies, the goal was to show that
regions of at least dozens of voxels are activated in response to certain stimuli (or movements),
using a block design (i.e., coarse-scale mapping). Trying to research the human brain at a finer
spatial resolution (using MVPA and RS, for example) and at a finer temporal resolution (fast
event related designs) is a much less explored territory (although these methods have become
66
more prevalent in the past year). More work is required to fully understand the extent to which
these methods reflect neuronal activity.
Directional selectivity and functional organization in other areas of the brain
In the first study, I showed that M1 voxels are directionally selective. However, I have found
additional directionally selective areas, such as PMd and SMA (although to a lesser extent; data
not shown). Fabbri and colleagues (2010) have also found directional selectivity in other areas
besides M1; including PMd, intraparietal sulcus (IPS) and the parietal reach region (PRR). This
raises questions regarding the interpretation of functional organization. Are other frontal and
parietal areas organized in the same way as M1? Directional tuning has been shown in
electrophysiological studies for neurons in PMd (Caminiti et al., 1991), PMv (Kakei et al., 2001),
cerebellum (Fortier et al., 1989) and area 5 (Kalaska et al., 1983). There is also some evidence
towards functional organization according to direction in PM (Stark et al., 2007). Perhaps this
directional selectivity is found in different areas for different reasons. Neurons could be
organized according to visual elements in parietal cortex, changing gradually into a motor
representation in the frontal cortex. I attempted to answer this question in my second study by
dissociating the visual and motor elements of the reaching task, utilizing the advantage of
scanning the whole brain, rather than just M1. However, I found no significant motor or visual
correlations for any area besides M1, despite a general trend we found in PMd, the cerebellum
and SMA. The statistical tests we used were extremely stringent and activation was not as strong
as in M1. It's possible that if instead of using fine joystick movement, we had used a
manipulandum typically used by the monkeys, or a motion-capture system, it would result in a
much more robust signal in areas beyond M1. A larger pool of subjects could have also given us
a clearer picture about other areas, perhaps a gradient of visual vs. motor representation.
Additionally, allowing extensive practice before the scan, thereby reducing the directional errors,
would help us achieve a better separation between visual and motor representations. Note that we
are not claiming that there is a stronger visual representation in M1 than in PM. The observation
of cursor motion resulted in no response. Our correlation analysis can produce significant results
67
only if the BOLD response is strong enough, which occurred only during movement. Therefore,
we cannot compare the visual and motor components of the different areas.
Functional Organization of Neurons in M1
In the first chapter I show that there is a functional organization according to movement direction
by showing that voxels respond differentially to movement direction. In the second chapter, this
differential response is shown to be related both to movement and to visual aspects.
Shen and Alexander (Shen and Alexander, 1997) showed that the same neurons can be sensitive
to the target at first, while preparing for movement, and then, during movement, become more
sensitive to movement direction. Based on these results, since we did not separate the different
trial epochs, we would expect a large overlap between the visual and the motor preferences
within a voxels. However, the overlap was no larger than expected by chance. The lack of a
subset of voxels that is informative about both visual and motor aspects also indicates that the
visually-selective and motor-selective neurons with the same directional preference are not in the
same cluster. Alternatively, if we imagine two separate populations of neurons; a visual
population (perhaps the more anterior neurons) and a motor population (the posterior neurons?),
we would expect two mutually exclusive groups of voxels. This option is also ruled out by our
results.
Since visual and motor groups of voxels were found to be independent (i.e., movement
selectivity of a voxel does not affect its probability to be visually-selective), the question of how
the clusters of the two modalities are spatially organized remains open. One possible explanation
is that the visually-selective neurons are at different cortical layers than the motor-selective
neurons, with each layer organized separately and independently. An alternative explanation is
that clusters of visually-selective neurons and of motor-selective neurons are interleaved within a
voxel. In this case, each voxel contains both visual and motor clusters. A voxel that is sensitive
to movement more than to visual aspects, for example, simply has more movement-selective
clusters. More research at the neuronal level is required to further address this question.
68
Another issue regarding functional organization is that at a very macroscopic level (cm) M1 is
organized according to body parts (Penfield and Boldrey, 1937; Woolsey et al., 1952). Within
the arm area, despite extensive overlap (Fetz and Cheney, 1980; Cheney and Fetz, 1985; Cheney
et al., 1985; Buys et al., 1986; Lemon et al., 1986; Fetz et al., 1989; Schieber and Hibbard, 1993
etc.), there is an organization according to fingers (Penfield and Rasmussen, 1950) and joints
(Murphy et al., 1978). How does this organization coincide with that according to movement
direction? Is M1 organized according to movement direction outside the arm area as well?
Furthermore, directionally tuned neurons were shown to change their PD while arm movements
in the same direction were made in different parts of extrapersonal space (Caminiti et al., 1990),
with different arm postures (Scott and Kalaska, 1995, 1997) and in different locations in the
workspace (Sergio and Kalaska, 1997, 2003). If neurons can change their PD under different
conditions, this might not be a basic property of the neurons. Does the entire cluster shift its PD?
How does this affect the organization? These questions remain to be answered.
Visual Representation in M1
In chapter 2 of the results we found a significant visual representation of the direction of motion,
which is in agreement with previous electrophysiological studies (Alexander and Crutcher, 1990;
Crutcher and Alexander, 1990; Hocherman and Wise, 1991; Lurito et al., 1991; Riehle et al.,
1994; Shen and Alexander, 1997). No activation was found in M1, however, when the task
required observation only even though the visual parameters were exactly the same as in the
movement task. Our results are in contradiction with many electrophysiological studies in
monkeys (Merchant et al., 2001; Musallam et al., 2004; Wahnoun et al., 2004; Santhanam et al.,
2006; Wahnoun et al., 2006; Velliste et al., 2008; Suminski et al., 2009; Dushanova and
Donoghue, 2010) and in humans with tetraplegia (Hochberg et al., 2006; Truccolo et al., 2008),
who did find observation-related activity in M1. Observation-related activity in M1 was also
found using TMS when it was associated with action (Petroni et al., 2010). It's likely that the
fMRI signal in an event-related design is too noisy to detect neuronal features that are seen in a
minority of the neuronal population, or perhaps the task given to the participants during the
observation task in order to keep them alert interrupted the association of the observed cursor
motion with hand movements.
69
Nevertheless, despite the lack of representation of observing cursor motion in M1, we are still
able to detect a correlate of target direction and/or cursor feedback. Thus, it is possible that what
we term "visual representation" is in fact a correlate of a higher order representation, beyond
simple observation. It could be related to gouging the error signal based on the visual feedback,
used to make online corrections and to make a more accurate movement in the next trials.
This possibility is in agreement with an fMRI study by Diedrichsen et al. (2005), that showed
strong M1 activation in response to both target errors (unpredictable changes in target location)
and execution errors (when prisms alter visual feedback or a force field alters limb dynamics).
Alternatively, the visual representation could stem from the representation of the goal of the task
in more general terms, namely to bring the cursor to the target by a hand movement in a different
direction. This possibility is consistent with several studies in monkeys that found M1 neurons
sensitive to visual features of the cue (Zhang et al., 1997b; Salinas and Romo, 1998; Zach et al.,
2008; Saleh et al., 2010) and with an fMRI study that found stronger M1 BOLD activation when
target distractors were present in a reach-to-grasp task (Chapman et al., 2007). Thus, M1 seems
to be involved in initial stages of movement planning.
As mentioned in chapter 2 of the results, it's also possible that the "visual representation" is
actually a representation of the baseline (unperturbed) mapping. If this is indeed the case, then
during the rotation run, the participant is making movements according to the novel mapping,
which would lead to "movement representation", while at the same time not fully suppressing the
previous mapping. This, in turn, would lead to lower, yet possibly significant, correlations
between the same target in the baseline and the rotation runs. Retention of the previous motor
programs has been shown in previous studies (Hadipour-Niktarash et al., 2007; Galea et al.,
2011).
Future Directions
It would be interesting to see whether the visual representation in M1 would translate into
a cue in a different modality, such as a sensory or an auditory cue. This might also help
distinguish between the cue and the visual feedback, and provide a more conclusive
answer as to which of them is responsible for the visual representation in M1.
70
In the discussion of the second chapter of the results, we bring up the possibility that our
results of a visual representation could in fact be a representation of novel mapping
(rotation) overriding the baseline mapping. In this case, the visual correlations we found
could actually stem from the underlying unexecuted motor program that is activated
when the cue is presented. This interpretation can be better accounted for in a task that is
not fast event-related, with longer trials. We could dissociate the planning from the final
stages of the reaching movement, where subjects are mostly fine tuning to accurately
reach the target. Such an experiment could also answer questions regarding the
visuomotor transformation throughout the trial. Of course, in this case there would be
significantly less trials in each direction, which would require decreasing noise, perhaps
by using a motion-capture system, which records the hand movement directly and is more
accurate than the MRI-compatible joystick.
Decoding movement direction at a single trial by implementing a classifier - This should
be done with a slow event-related design (also using a motion-capture system), where
deconvolution is not necessary to extract the activation of each condition, and separation
between activations of the different trials is much more straightforward. Successful
decoding could bring us closer to a brain-machine interface, which would help predict the
intended direction of hand movement of a paralyzed person and translate these intentions
into movement of a robotic arm. The advantage is that fMRI is not invasive. However, it
would be difficult to translate it into everyday life, since a person has to lie inside a
scanner without making any head movements.
It would be advantageous to study RS in single neurons, MUA, LFP and fMRI in
macaques that did a task with a clear adapter and test trials, with shorter time intervals
before the test trials. Such a task would be the optimal way to compare the results with
previous visual adaptation studies. In addition, the LFP analysis was done only at the
time domain. De Baene and Vogels (2010) found no significant RS in IT in the time
domain, while they did find RS at the gamma range in the frequency domain. It remains
to be seen whether there is RS at the gamma domain in M1.
Last, we can test whether there is more suppression of the fMRI signal in response to
novel movements. For example, we can have subjects practice movements only to
horizontal directions, and then see if there is a difference in RS to horizontal and to
71
vertical movements. Alternatively, we could look at more complex movements, by
having subjects practice specific sequences of several reaching movements in a row, and
then see whether there is more RS to novel sequences than to the practiced ones.
72
References
Aguirre G, Zarahn E, D'esposito M (1998) The variability of human, BOLD hemodynamic responses.
Neuroimage 8:360-369.
Albright TD, Desimone R, Gross CG (1984) Columnar Organization of Directionally Selective Cells in Visual
Area Mt of the Macaque. Journal of Neurophysiology 51:16-31.
Alexander GE, Crutcher MD (1990) Neural representations of the target (goal) of visually guided arm
movements in three motor areas of the monkey. Journal of neurophysiology 64:164.
Amirikian B, Georgopulos AP (2000) Directional tuning profiles of motor cortical cells. Neuroscience
research 36:73-79.
Amirikian B, Georgopoulos AP (2003) Modular organization of directionally tuned cells in the motor
cortex: Is there a short-range order? Proceedings of the National Academy of Sciences
100:12474.
Andersen R, Essick G, Siegel R (1987) Neurons of area 7 activated by both visual stimuli and oculomotor
behavior. Experimental Brain Research 67:316-322.
Asanuma H (1975) Recent developments in the study of the columnar arrangement of neurons within
the motor cortex. Physiological reviews 55:143-156.
Asanuma H, Ward J (1971) Patterns of contraction of distal forelimb muscles produced by intracortical
stimulation in cats. Brain Res 27:97-109.
Asanuma H, Rosen I (1972) Topographical organization of cortical efferent zones projecting to distal
forelimb muscles in the monkey. Experimental Brain Research 14:243-256.
Avidan G, Hasson U, Hendler T, Zohary E, Malach R (2002) Analysis of the neuronal selectivity underlying
low fMRI signals. Current Biology 12:964-972.
Beisteiner R, Windischberger C, Lanzenberger R, Edward V, Cunnington R, Erdler M, Gartus A, Streibl B,
Moser E, Deecke L (2001) Finger somatotopy in human motor cortex. Neuroimage 13:10161026.
Ben-Shaul Y, Stark E, Asher I, Drori R, Nadasdy Z, Abeles M (2003) Dynamical organization of directional
tuning in the primate premotor and primary motor cortex. Journal of Neurophysiology 89:11361142.
Berens P, Keliris G, Ecker A, Logothetis N, Tolias A (2008) Feature selectivity of the gamma-band of the
local field potential in primate primary visual cortex. Frontiers in Neuroscience 2:199.
Blasdel GG, Salama G (1986) Voltage-sensitive dyes reveal a modular organization in monkey striate
cortex. Nature 321:579-585.
Bonhoeffer T, Grinvald A (1991) Iso-orientation domains in cat visual cortex are arranged in pinwheellike patterns. Nature 353:429-431.
Bortoff GA, Strick P (1993) Corticospinal terminations in two new-world primates: further evidence that
corticomotoneuronal connections provide part of the neural substrate for manual dexterity. The
Journal of Neuroscience 13:5105-5118.
Boussaoud D, Wise S (1993a) Primate frontal cortex: effects of stimulus and movement. Experimental
Brain Research 95:28-40.
Boussaoud D, Wise SP (1993b) Primate frontal cortex: neuronal activity following attentional versus
intentional cues. Experimental Brain Research 95:15-27.
Boynton GM (2005) Imaging orientation selectivity: decoding conscious perception in V1. Nature
Neuroscience 8:541-542.
73
Brants M, Baeck A, Wagemans J, de Beeck HPO (2011) Multiple scales of organization for object
selectivity in ventral visual cortex. Neuroimage.
Brinkman C (1981) Lesions in supplementary motor area interfere with a monkey's performance of a
bimanual coordination task. Neuroscience letters 27:267-270.
Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, Schacter D, Rosen B, Dale AM (1998)
Functional-anatomic correlates of object priming in humans revealed by rapid presentation
event-related fMRI. Neuron 20:285-296.
Buys E, Lemon R, Mantel G, Muir R (1986) Selective facilitation of different hand muscles by single
corticospinal neurones in the conscious monkey. The Journal of physiology 381:529-549.
Caminiti R, Johnson P, Urbano A (1990) Making arm movements within different parts of space: dynamic
aspects in the primate motor cortex. The Journal of Neuroscience 10:2039.
Caminiti R, Johnson P, Galli C, Ferraina S, Burnod Y (1991) Making arm movements within different parts
of space: the premotor and motor cortical representation of a coordinate system for reaching to
visual targets. The Journal of Neuroscience 11:1182.
Campbell AW (1905) Histological studies on the localisation of cerebral function: University Press.
Chander D, Chichilnisky E (2001) Adaptation to temporal contrast in primate and salamander retina. The
Journal of Neuroscience 21:9904.
Chapman H, Pierno AC, Cunnington R, Gavrilescu M, Egan G, Castiello U (2007) The neural basis of
selection-for-action. Neuroscience letters 417:171.
Cheney PD, Fetz EE (1985) Comparable patterns of muscle facilitation evoked by individual
corticomotoneuronal (CM) cells and by single intracortical microstimuli in primates: evidence for
functional groups of CM cells. Journal of neurophysiology 53:786-804.
Cheney PD, Fetz EE, Palmer SS (1985) Patterns of facilitation and suppression of antagonist forelimb
muscles from motor cortex sites in the awake monkey. Journal of neurophysiology 53:805-820.
Cheyne D, Kristeva R, Deecke L (1991) Homuncular organization of human motor cortex as indicated by
neuromagnetic recordings. Neuroscience letters 122:17-20.
Colebatch J, Deiber M, Passingham R, Friston K, Frackowiak R (1991) Regional cerebral blood flow during
voluntary arm and hand movements in human subjects. Journal of neurophysiology 65:13921401.
Conner CR, Ellmore TM, Pieters TA, DiSano MA, Tandon N (2011) Variability of the Relationship between
Electrophysiology and BOLD-fMRI across Cortical Regions in Humans. The Journal of
Neuroscience 31:12855-12865.
Crammond D, Kalaska J (1994) Modulation of preparatory neuronal activity in dorsal premotor cortex
due to stimulus-response compatibility. Journal of neurophysiology 71:1281-1284.
Crammond DJ, Kalaska JF (1996) Differential relation of discharge in primary motor cortex and premotor
cortex to movements versus actively maintained postures during a reaching task. Experimental
Brain Research 108:45-61.
Crutcher MD, Alexander GE (1990) Movement-related neuronal activity selectively coding either
direction or muscle pattern in three motor areas of the monkey. Journal of neurophysiology
64:151-163.
Dale A (1999) Optimal experimental design for event-related fMRI. Human Brain Mapping 8:109-114.
De Baene W, Vogels R (2010) Effects of adaptation on the stimulus selectivity of macaque inferior
temporal spiking activity and local field potentials. Cerebral Cortex 20:2145.
Dechent P, Frahm J (2003) Functional somatotopy of finger representations in human primary motor
cortex. Human brain mapping 18:272-283.
Desimone R (1996) Neural mechanisms for visual memory and their role in attention. Proceedings of the
National Academy of Sciences 93:13494-13499.
74
di Pellegrino G, Wise SP (1993) Visuospatial versus visuomotor activity in the premotor and prefrontal
cortex of a primate. The Journal of Neuroscience 13:1227-1243.
Diedrichsen J, Hashambhoy Y, Rane T, Shadmehr R (2005) Neural correlates of reach errors. The Journal
of Neuroscience 25:9919-9931.
Dinstein I, Hasson U, Rubin N, Heeger DJ (2007) Brain areas selective for both observed and executed
movements. Journal of neurophysiology 98:1415.
Dushanova J, Donoghue J (2010) Neurons in primary motor cortex engaged during action observation.
European Journal of Neuroscience 31:386-398.
Eisenberg M, Shmuelof L, Vaadia E, Zohary E (2010) Functional organization of human motor cortex:
directional selectivity for movement. The Journal of Neuroscience 30:8897.
Enroth-Cugell C, Shapley R (1973) Adaptation and dynamics of cat retinal ganglion cells. The Journal of
Physiology 233:271.
Evarts EV (1968) Relation of pyramidal tract activity to force exerted during voluntary movement. J
Neurophysiol 31:14-27.
Fabbri S, Caramazza A, Lingnau A (2010) Tuning Curves for Movement Direction in the Human
Visuomotor System. The Journal of Neuroscience 30:13488.
Ferrier D (1873) Experimental researches in cerebral physiology and pathology. Journal of anatomy and
physiology 8:152.
Fetz E, Cheney P, Mewes K, Palmer S (1989) Control of forelimb muscle activity by populations of
corticomotoneuronal and rubromotoneuronal cells. Progress in brain research 80:437-449.
Fetz EE, Cheney PD (1980) Postspike facilitation of forelimb muscle activity by primate
corticomotoneuronal cells. Journal of neurophysiology 44:751-772.
Filimon F, Nelson JD, Hagler DJ, Sereno MI (2007) Human cortical representations for reaching: mirror
neurons for execution, observation, and imagery. Neuroimage 37:1315-1328.
Fogassi L, Gallese V, Fadiga L, Luppino G, Matelli M, Rizzolatti G (1996) Coding of peripersonal space in
inferior premotor cortex (area F4). Journal of neurophysiology 76:141-157.
Fortier PA, Kalaska JF, Smith AM (1989) Cerebellar neuronal activity related to whole-arm reaching
movements in the monkey. Journal of neurophysiology 62:198.
Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ (1994) Statistical parametric maps
in functional imaging: a general linear approach. Human brain mapping 2:189-210.
Fritsch GT, Hitzig E (1870) Ueber die elektrische Erregbarkeit des Grosshirns: Veit.
Gail A, Brinksmeyer HJ, Eckhorn R (2003) Simultaneous mapping of binocular and monocular receptive
fields in awake monkeys for calibrating eye alignment in a dichoptical setup. Journal of
neuroscience methods 126:41-56.
Galea JM, Vazquez A, Pasricha N, de Xivry JJO, Celnik P (2011) Dissociating the roles of the cerebellum
and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns.
Cerebral Cortex 21:1761-1770.
Gallese V, Fadiga L, Fogassi L, Rizzolatti G (1996) Action recognition in the premotor cortex. Brain
119:593-609.
Gardner JL (2010) Is cortical vasculature functionally organized? Neuroimage 49:1953-1956.
Gardner JL, Sun P, Waggoner RA, Ueno K, Tanaka K, Cheng K (2005) Contrast adaptation and
representation in human early visual cortex. Neuron 47:607-620.
Gaymard B, Pierrot‐Deseilligny C, Rivaud S (1990) Impairment of sequences of memory‐guided saccades
after supplementary motor area lesions. Annals of neurology 28:622-626.
Georgopoulos AP, Schwartz AB, Kettner RE (1986) Neuronal population coding of movement direction.
Science 233:1416.
75
Georgopoulos AP, Kettner RE, Schwartz AB (1988) Primate motor cortex and free arm movements to
visual targets in three-dimensional space. II. Coding of the direction of movement by a neuronal
population. The Journal of Neuroscience 8:2928.
Georgopoulos AP, Taira M, Lukashin A (1993) Cognitive neurophysiology of the motor cortex. Science
260:47.
Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT (1982) On the Relations between the Direction of
Two-Dimensional Arm Movements and Cell Discharge in Primate Motor Cortex. J Neurosci
2:1527-1537.
Gerloff C, Corwell B, Chen R, Hallett M, Cohen LG (1997) Stimulation over the human supplementary
motor area interferes with the organization of future elements in complex motor sequences.
Brain 120:1587-1602.
Gieselmann M, Thiele A (2008) Comparison of spatial integration and surround suppression
characteristics in spiking activity and the local field potential in macaque V1. European Journal
of Neuroscience 28:447-459.
Glover GH (1999) Deconvolution of Impulse Response in Event-Related BOLD fMRI< sup> 1</sup>.
Neuroimage 9:416-429.
Godschalk M, Lemon R, Kuypers H, Van Der Steen J (1985) The involvement of monkey premotor cortex
neurones in preparation of visually cued arm movements. Behavioural brain research 18:143157.
Grafton ST, Woods RP, Mazziotta JC, Phelps ME (1991) Somatotopic mapping of the primary motor
cortex in humans: activation studies with cerebral blood flow and positron emission
tomography. Journal of neurophysiology 66:735-743.
Grill-Spector K, Malach R (2001) fMR-adaptation: a tool for studying the functional properties of human
cortical neurons. Acta Psychologica 107:293-321.
Grill-Spector K, Henson R, Martin A (2006) Repetition and the brain: neural models of stimulus-specific
effects. Trends in Cognitive Sciences 10:14-23.
Grill-Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R (1999) Differential processing of
objects under various viewing conditions in the human lateral occipital complex. Neuron 24:187203.
Hadipour-Niktarash A, Lee CK, Desmond JE, Shadmehr R (2007) Impairment of retention but not
acquisition of a visuomotor skill through time-dependent disruption of primary motor cortex.
The Journal of Neuroscience 27:13413-13419.
Halsband U, Matsuzaka Y, Tanji J (1994) Neuronal activity in the primate supplementary, presupplementary and premotor cortex during externally and internally instructed sequential
movements. Neuroscience research 20:149-155.
Hatsopoulos NG (2010) Columnar organization in the motor cortex. Cortex; a journal devoted to the
study of the nervous system and behavior 46:270.
Hatsopoulos NG, Ojakangas CL, Paninski L, Donoghue JP (1998) Information about movement direction
obtained from synchronous activity of motor cortical neurons. Proceedings of the National
Academy of Sciences 95:15706.
Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P (2001) Distributed and overlapping
representations of faces and objects in ventral temporal cortex. Science 293:2425.
Haynes JD, Rees G (2005) Predicting the orientation of invisible stimuli from activity in human primary
visual cortex. Nature Neuroscience 8:686-691.
He S, Dum R, Strick P (1995) Topographic organization of corticospinal projections from the frontal lobe:
motor areas on the medial surface of the hemisphere. The Journal of Neuroscience 15:32843306.
76
He SQ, Dum R, Strick P (1993) Topographic organization of corticospinal projections from the frontal
lobe: motor areas on the lateral surface of the hemisphere. The Journal of Neuroscience 13:952980.
Heeger DJ, Huk AC, Geisler WS, Albrecht DG (2000) Spikes versus BOLD: what does neuroimaging tell us
about neuronal activity? Nat Neurosci 3:631-632.
Henson R, Rugg M (2003) Neural response suppression, haemodynamic repetition effects, and
behavioural priming. Neuropsychologia 41:263-270.
Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD,
Donoghue JP (2006) Neuronal ensemble control of prosthetic devices by a human with
tetraplegia. Nature 442:164-171.
Hocherman S, Wise S (1991) Effects of hand movement path on motor cortical activity in awake,
behaving rhesus monkeys. Experimental Brain Research 83:285-302.
Hubel DH, Wiesel TN (1962) Receptive fields, binocular interaction and functional architecture in the
cat's visual cortex. J Physiol 160:106-154.
Huntley GW, Jones EG (1991) Relationship of intrinsic connections to forelimb movement
representations in monkey motor cortex: a correlative anatomic and physiological study. Journal
of neurophysiology 66:390-413.
Imig TJ, Adrian HO (1977) Binaural Columns in Primary Field (A1) of Cat Auditory-Cortex. Brain Res
138:241-257.
James TW, Gauthier I (2006) Repetition‐induced changes in BOLD response reflect accumulation of
neural activity. Human brain mapping 27:37-46.
Jeannerod M, Arbib MA, Rizzolatti G, Sakata H (1995) Grasping objects: the cortical mechanisms of
visuomotor transformation. Trends in Neurosciences 18:314-320.
Jenkins I, Brooks D, Nixon P, Frackowiak R, Passingham R (1994) Motor sequence learning: a study with
positron emission tomography. The Journal of Neuroscience 14:3775-3790.
Johnson PB, Ferraina S, Bianchi L, Caminiti R (1996) Cortical networks for visual reaching: physiological
and anatomical organization of frontal and parietal lobe arm regions. Cerebral Cortex 6:102.
Kakei S, Hoffman DS, Strick PL (1999) Muscle and movement representations in the primary motor
cortex. Science 285:2136.
Kakei S, Hoffman DS, Strick PL (2001) Direction of action is represented in the ventral premotor cortex.
Nat Neurosci 4:1020-1025.
Kalaska J, Caminiti R, Georgopoulos AP (1983) Cortical mechanisms related to the direction of twodimensional arm movements: relations in parietal area 5 and comparison with motor cortex.
Experimental Brain Research 51:247-260.
Kaliukhovich DA, Vogels R (2011) Stimulus repetition probability does not affect repetition suppression
in macaque inferior temporal cortex. Cerebral Cortex 21:1547.
Kamitani Y, Tong F (2005) Decoding the visual and subjective contents of the human brain. Nature
Neuroscience 8:679-685.
Katzner S, Nauhaus I, Benucci A, Bonin V, Ringach DL, Carandini M (2009) Local origin of field potentials
in visual cortex. Neuron 61:35-41.
Kleinschmidt A, Nitschke MF, Frahm J (2006) Somatotopy in the human motor cortex hand area. A
high‐resolution functional MRI study. European Journal of Neuroscience 9:2178-2186.
Kohn A, Movshon JA (2004) Adaptation changes the direction tuning of macaque MT neurons. Nature
Neuroscience 7:764-772.
Kourtzi Z, Kanwisher N (2001) Representation of perceived object shape by the human lateral occipital
complex. Science 293:1506.
Kreiman G, Hung CP, Kraskov A, Quiroga RQ, Poggio T, DiCarlo JJ (2006) Object selectivity of local field
potentials and spikes in the macaque inferior temporal cortex. Neuron 49:433-445.
77
Krekelberg B, Boynton GM, van Wezel RJA (2006) Adaptation: from single cells to BOLD signals. TRENDS
in Neurosciences 29:250-256.
Kruse W, Eckhorn R (1996) Inhibition of sustained gamma oscillations (35-80 Hz) by fast transient
responses in cat visual cortex. Proceedings of the National Academy of Sciences 93:6112-6117.
Kurata K, Tanji J (1986) Premotor cortex neurons in macaques: activity before distal and proximal
forelimb movements. The Journal of Neuroscience 6:403-411.
Kurata K, Wise S (1988) Premotor cortex of rhesus monkeys: set-related activity during two conditional
motor tasks. Experimental Brain Research 69:327-343.
Kurata K, Hoffman DS (1994) Differential effects of muscimol microinjection into dorsal and ventral
aspects of the premotor cortex of monkeys. Journal of neurophysiology 71:1151-1164.
Kwan HC, Murphy JT, Wong YC (1987) Interaction between neurons in precentral cortical zones
controlling different joints. Brain Res 400:259-269.
Larsson J, Smith AT (2012) FMRI repetition suppression: neuronal adaptation or stimulus expectation?
Cerebral Cortex 22:567-576.
Lee D, Quessy S (2003) Activity in the supplementary motor area related to learning and performance
during a sequential visuomotor task. Journal of neurophysiology 89:1039-1056.
Lee D, Port NL, Kruse W, Georgopoulos AP (1998) Variability and correlated noise in the discharge of
neurons in motor and parietal areas of the primate cortex. The Journal of Neuroscience 18:1161.
Lemon R (1981) Variety of functional organization within the monkey motor cortex. The Journal of
physiology 311:521-540.
Lemon R, Mantel G, Muir R (1986) Corticospinal facilitation of hand muscles during voluntary movement
in the conscious monkey. The Journal of physiology 381:497-527.
Leyton A, Sherrington CS (1917) Observations on the excitable cortex of the chimpanzee, orang-utan,
and gorilla. Experimental Physiology 11:135-222.
Li L, Miller EK, Desimone R (1993) The representation of stimulus familiarity in anterior inferior temporal
cortex. Journal of neurophysiology 69:1918-1929.
Liu J, Newsome WT (2006) Local field potential in cortical area MT: stimulus tuning and behavioral
correlations. The Journal of Neuroscience 26:7779-7790.
Logothetis NK, Kayser C, Oeltermann A (2007) In vivo measurement of cortical impedance spectrum in
monkeys: implications for signal propagation. Neuron 55:809-823.
Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A (2001) Neurophysiological investigation of
the basis of the fMRI signal. Nature 412:150-157.
Lurito J, Georgakopoulos T, Georgopoulos A (1991) Cognitive spatial-motor processes. Experimental
Brain Research 87:562-580.
Malach R (2012) Targeting the functional properties of cortical neurons using fMR-adaptation.
Neuroimage.
Martin J, Ghez C (1985) Task-related coding of stimulus and response in cat motor cortex. Experimental
Brain Research 57:427-442.
Matsuzaka Y, Aizawa H, Tanji J (1992) A motor area rostral to the supplementary motor area
(presupplementary motor area) in the monkey: neuronal activity during a learned motor task.
Journal of neurophysiology 68:653-662.
Mauritz KH, Wise S (1986) Premotor cortex of the rhesus monkey: neuronal activity in anticipation of
predictable environmental events. Experimental Brain Research 61:229-244.
Maynard E, Hatsopoulos N, Ojakangas C, Acuna B, Sanes J, Normann R, Donoghue J (1999) Neuronal
interactions improve cortical population coding of movement direction. The Journal of
Neuroscience 19:8083.
Mehring C, Rickert J, Vaadia E, de Oliveira SC, Aertsen A, Rotter S (2003) Inference of hand movements
from local field potentials in monkey motor cortex. Nat Neurosci 6:1253-1254.
78
Meier JD, Aflalo TN, Kastner S, Graziano MSA (2008) Complex organization of human primary motor
cortex: a high-resolution fMRI study. Journal of neurophysiology 100:1800-1812.
Merchant H, Battaglia-Mayer A, Georgopoulos A (2001) Effects of optic flow in motor cortex and area
7a. Journal of neurophysiology 86:1937-1954.
Miller EK, Desimone R (1994) Parallel neuronal mechanisms for short-term memory. Science-AAASWeekly Paper Edition-including Guide to Scientific Information 263:520-522.
Mitzdorf U (1987) Properties of the evoked potential generators: current source-density analysis of
visually evoked potentials in the cat cortex. International Journal of Neuroscience 33:33-59.
Movshon JA, Lennie P (1979) Pattern-selective adaptation in visual cortical neurones. Nature 278:850852.
Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, Malach R (2005) Coupling between neuronal firing,
field potentials, and FMRI in human auditory cortex. Science 309:951.
Müller JR, Metha AB, Krauskopf J, Lennie P (1999) Rapid adaptation in visual cortex to the structure of
images. Science 285:1405.
Murphy J, Kwan H, Wong Y (1985) Cross correlation studies in primate motor cortex: synaptic
interaction and shared input. The Canadian journal of neurological sciences Le journal canadien
des sciences neurologiques 12:11.
Murphy J, Kwan H, MacKay W, Wong Y (1978) Spatial organization of precentral cortex in awake
primates. III. Input-output coupling. Journal of neurophysiology 41:1132-1139.
Musallam S, Corneil B, Greger B, Scherberger H, Andersen R (2004) Cognitive control signals for neural
prosthetics. Science 305:258-262.
Mushiake H, Inase M, Tanji J (1990) Selective coding of motor sequence in the supplementary motor
area of the monkey cerebral cortex. Experimental Brain Research 82:208-210.
Mushiake H, Inase M, Tanji J (1991) Neuronal activity in the primate premotor, supplementary, and
precentral motor cortex during visually guided and internally determined sequential
movements. Journal of neurophysiology 66:705-718.
Nir Y, Fisch L, Mukamel R, Gelbard-Sagiv H, Arieli A, Fried I, Malach R (2007) Coupling between neuronal
firing rate, gamma LFP, and BOLD fMRI is related to interneuronal correlations. Current Biology
17:1275-1285.
Ohki K, Chung S, Ch'ng YH, Kara P, Reid RC (2005) Functional imaging with cellular resolution reveals
precise micro-architecture in visual cortex. Nature 433:597-603.
Op de Beeck HP (2010) Against hyperacuity in brain reading: Spatial smoothing does not hurt
multivariate fMRI analyses? Neuroimage 49:1943-1948.
Passingham R (1988) Premotor cortex and preparation for movement. Experimental Brain Research
70:590-596.
Paz R, Boraud T, Natan C, Bergman H, Vaadia E (2003) Preparatory activity in motor cortex reflects
learning of local visuomotor skills. Nature Neuroscience 6:882-890.
Pedreira C, Mormann F, Kraskov A, Cerf M, Fried I, Koch C, Quiroga RQ (2010) Responses of human
medial temporal lobe neurons are modulated by stimulus repetition. Journal of neurophysiology
103:97.
Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G (1992) Understanding motor events: a
neurophysiological study. Experimental Brain Research 91:176-180.
Penfield W, Boldrey E (1937) Somatic motor and sensory representation in the cerebral cortex of man as
studied by electrical stimulation. Brain: A journal of neurology.
Penfield W, Rasmussen T (1950) The cerebral cortex of man: a clinical study of localization of function:
Macmillan.
Petroni A, Baguear F, Della-Maggiore V (2010) Motor resonance may originate from sensorimotor
experience. Journal of neurophysiology 104:1867-1871.
79
Powell T, Mountcastle V (1959) Some aspects of the functional organization of the cortex of the
postcentral gyrus of the monkey: a correlation of findings obtained in a single unit analysis with
cytoarchitecture. Bulletin of the Johns Hopkins Hospital 105:133.
Rao SM, Binder J, Hammeke T, Bandettini P, Bobholz J, Frost J, Myklebust B, Jacobson R, Hyde J (1995)
Somatotopic mapping of the human primary motor cortex with functional magnetic resonance
imaging. Neurology 45:919-924.
Raos V, Umiltá MA, Murata A, Fogassi L, Gallese V (2006) Functional properties of grasping-related
neurons in the ventral premotor area F5 of the macaque monkey. Journal of neurophysiology
95:709.
Rasch M, Logthetis NK, Kreiman G (2009) From Neurons to Circuits: Linear Estimation of Local Field
Potentials. J Neurosci 29:13785-13796.
Rauch A, Rainer G, Logothetis NK (2008) The effect of a serotonin-induced dissociation between spiking
and perisynaptic activity on BOLD functional MRI. Proceedings of the National Academy of
Sciences 105:6759.
Rickert J, de Oliveira SC, Vaadia E, Aertsen A, Rotter S, Mehring C (2005) Encoding of movement
direction in different frequency ranges of motor cortical local field potentials. J Neurosci
25:8815-8824.
Riehle A, Requin J (1989) Monkey primary motor and premotor cortex: single-cell activity related to
prior information about direction and extent of an intended movement. Journal of
neurophysiology 61:534-549.
Riehle A, Kornblum S, Requin J (1994) Neuronal coding of stimulus-response association rules in the
motor cortex. Neuroreport: An International Journal for the Rapid Communication of Research
in Neuroscience.
Rizzolatti G, Camarda R, Fogassi L, Gentilucci M, Luppino G, Matelli M (1988) Functional organization of
inferior area 6 in the macaque monkey. Experimental Brain Research 71:491-507.
Roland PE, Larsen B, Lassen N, Skinhoj E (1980) Supplementary motor area and other cortical areas in
organization of voluntary movements in man. Journal of neurophysiology 43:118-136.
Rothschild G, Nelken I, Mizrahi A (2010) Functional organization and population dynamics in the mouse
primary auditory cortex. Nat Neurosci 13:353-360.
Saleh M, Reimer J, Penn R, Ojakangas CL, Hatsopoulos NG (2010) Fast and slow oscillations in human
primary motor cortex predict oncoming behaviorally relevant cues. Neuron 65:461-471.
Salenius S, Portin K, Kajola M, Salmelin R, Hari R (1997) Cortical control of human motoneuron firing
during isometric contraction. Journal of neurophysiology 77:3401-3405.
Salinas E, Romo R (1998) Conversion of sensory signals into motor commands in primary motor cortex.
The Journal of Neuroscience 18:499-511.
Sanes JN, Donoghue JP, Thangaraj V, Edelman RR, Warach S (1995) Shared neural substrates controlling
hand movements in human motor cortex. SCIENCE-NEW YORK THEN WASHINGTON-:1775-1775.
Santhanam G, Ryu SI, Byron MY, Afshar A, Shenoy KV (2006) A high-performance brain–computer
interface. Nature 442:195-198.
Sapountzis P, Schluppeck D, Bowtell R, Peirce JW (2010) A comparison of fMRI adaptation and
multivariate pattern classification analysis in visual cortex. Neuroimage 49:1632-1640.
Sawamura H, Orban GA, Vogels R (2006) Selectivity of neuronal adaptation does not match response
selectivity: a single-cell study of the FMRI adaptation paradigm. Neuron 49:307-318.
Sawamura H, Georgieva S, Vogels R, Vanduffel W, Orban GA (2005) Using functional magnetic resonance
imaging to assess adaptation and size invariance of shape processing by humans and monkeys.
The Journal of Neuroscience 25:4294-4306.
Schaal S, Sternad D, Osu R, Kawato M (2004) Rhythmic arm movement is not discrete. Nat Neurosci
7:1136-1143.
80
Schacter DL, Buckner RL, Koutstaal W, Dale AM, Rosen BR (1997) Late onset of anterior prefrontal
activity during true and false recognition: an event-related fMRI study. Neuroimage 6:259-269.
Schieber MH, Hibbard LS (1993) How somatotopic is the motor cortex hand area? Science; Science.
Scott SH, Kalaska JF (1995) Changes in motor cortex activity during reaching movements with similar
hand paths but different arm postures. Journal of neurophysiology 73:2563.
Scott SH, Kalaska JF (1997) Reaching movements with similar hand paths but different arm orientations.
I. Activity of individual cells in motor cortex. Journal of neurophysiology 77:826.
Sergio LE, Kalaska JF (1997) Systematic changes in directional tuning of motor cortex cell activity with
hand location in the workspace during generation of static isometric forces in constant spatial
directions. Journal of neurophysiology 78:1170.
Sergio LE, Kalaska JF (2003) Systematic changes in motor cortex cell activity with arm posture during
directional isometric force generation. Journal of neurophysiology 89:212.
Serrien DJ, Strens LHA, Oliviero A, Brown P (2002) Repetitive transcranial magnetic stimulation of the
supplementary motor area (SMA) degrades bimanual movement control in humans.
Neuroscience letters 328:89-92.
Sharpee TO, Sugihara H, Kurgansky AV, Rebrik SP, Stryker MP, Miller KD (2006) Adaptive filtering
enhances information transmission in visual cortex. Nature 439:936.
Shen L, Alexander GE (1997) Neural correlates of a spatial sensory-to-motor transformation in primary
motor cortex. Journal of neurophysiology 77:1171.
Shima K, Tanji J (1998) Both supplementary and presupplementary motor areas are crucial for the
temporal organization of multiple movements. Journal of neurophysiology 80:3247-3260.
Shinoda Y, Yokota JI, Futami T (1981) Divergent projection of individual corticospinal axons to
motoneurons of multiple muscles in the monkey. Neuroscience letters 23:7-12.
Shmuel A, Augath M, Oeltermann A, Logothetis NK (2006) Negative functional MRI response correlates
with decreases in neuronal activity in monkey visual area V1. Nat Neurosci 9:569-577.
Sirotin YB, Das A (2009) Anticipatory haemodynamic signals in sensory cortex not predicted by local
neuronal activity. Nature 457:475-479.
Smirnakis SM, Berry MJ, Warland DK, Bialek W, Meister M (1997) Adaptation of retinal processing to
image contrast and spatial scale.
Sobotka S, Ringo JL (1996) Mnemonic responses of single units recorded from monkey inferotemporal
cortex, accessed via transcommissural versus direct pathways: a dissociation between unit
activity and behavior. The Journal of Neuroscience 16:4222-4230.
Solomon SG, Peirce JW, Dhruv NT, Lennie P (2004) Profound contrast adaptation early in the visual
pathway. Neuron 42:155-162.
Stark E, Asher I, Abeles M (2007) Encoding of reach and grasp by single neurons in premotor cortex is
independent of recording site. Journal of neurophysiology 97:3351.
Stark E, Drori R, Abeles M (2009) Motor cortical activity related to movement kinematics exhibits local
spatial organization. Cortex 45:418-431.
Suminski AJ, Tkach DC, Hatsopoulos NG (2009) Exploiting multiple sensory modalities in brain-machine
interfaces. Neural networks: the official journal of the International Neural Network Society
22:1224.
Summerfield C, Monti JMP, Trittschuh EH, Mesulam MM, Egner T (2008) Neural repetition suppression
reflects fulfilled perceptual expectations. Nature Neuroscience 11:1004.
Thach W (1978) Correlation of neural discharge with pattern and force of muscular activity, joint
position, and direction of intended next movement in motor cortex and cerebellum. Journal of
neurophysiology 41:654.
81
Tootell RBH, Hadjikhani NK, Vanduffel W, Liu AK, Mendola JD, Sereno MI, Dale AM (1998) Functional
analysis of primary visual cortex (V1) in humans. Proceedings of the National Academy of
Sciences 95:811.
Truccolo W, Friehs GM, Donoghue JP, Hochberg LR (2008) Primary motor cortex tuning to intended
movement kinematics in humans with tetraplegia. The Journal of Neuroscience 28:1163-1178.
Vaadia E, Haalman I, Abeles M, Bergman H, Prut Y, Slovin H, Aertsen A (1995) Dynamics of neuronal
interactions in monkey cortex in relation to behavioural events. Nature 373:515-518.
Velliste M, Perel S, Spalding MC, Whitford AS, Schwartz AB (2008) Cortical control of a prosthetic arm for
self-feeding. Nature 453:1098-1101.
Victor JD, Purpura K, Katz E, Mao B (1994) Population encoding of spatial frequency, orientation, and
color in macaque V1. Journal of neurophysiology 72:2151-2166.
Wahnoun R, Tillery SIH, He J (2004) Neuron selection and visual training for population vector based
cortical control. In: Engineering in Medicine and Biology Society, 2004. IEMBS'04. 26th Annual
International Conference of the IEEE, pp 4607-4610: IEEE.
Wahnoun R, He J, Tillery SIH (2006) Selection and parameterization of cortical neurons for
neuroprosthetic control. Journal of neural engineering 3:162.
Weinrich M, Wise SP (1982) The premotor cortex of the monkey. The Journal of Neuroscience 2:1329.
Weinrich M, Wise S, Mauritz KH (1984) A neurophysiological study of the premotor cortex in the rhesus
monkey. Brain 107:385-414.
Wiggs CL, Martin A (1998) Properties and mechanisms of perceptual priming. Current opinion in
neurobiology 8:227-233.
Wise S, Mauritz KH (1985) Set-related neuronal activity in the premotor cortex of rhesus monkeys:
effects of changes in motor set. Proceedings of the Royal society of London Series B Biological
sciences 223:331-354.
Woolsey CN, Settlage PH, Meyer DR, Sencer W, PINTO HT, Travis AM (1952) Patterns of localization in
precentral and" supplementary" motor areas and their relation to the concept of a premotor
area. Research publications-Association for Research in Nervous and Mental Disease 30:238.
Xing D, Yeh CI, Shapley RM (2009) Spatial spread of the local field potential and its laminar variation in
visual cortex. The Journal of Neuroscience 29:11540-11549.
Zach N, Inbar D, Grinvald Y, Bergman H, Vaadia E (2008) Emergence of novel representations in primary
motor cortex and premotor neurons during associative learning. The Journal of Neuroscience
28:9545.
Zeharia N, Hertz U, Flash T, Amedi A (2012) Negative blood oxygenation level dependent homunculus
and somatotopic information in primary motor cortex and supplementary motor area.
Proceedings of the National Academy of Sciences 109:18565-18570.
Zhang J, Riehle A, Requin J, Kornblum S (1997a) Dynamics of single neuron activity in monkey primary
motor cortex related to sensorimotor transformation. The Journal of Neuroscience 17:2227.
Zhang J, Riehle A, Requin J, Kornblum S (1997b) Dynamics of single neuron activity in monkey primary
motor cortex related to sensorimotor transformation. The Journal of Neuroscience 17:22272246.
82
Supplementary Data for Results I
ארגון פונקציונאלי בקליפת המוח המוטורית
חיבור לשם קבלת תואר דוקטור לפילוסופיה
מאת
מיכל אייזנברג
הוגש לסנט האוניברסיטה העברית בירושלים
אפריל 2102
עבודה זו נעשתה בהדרכתם של
פרופ' אהוד זהרי
ופרופ' אילון ועדיה
תקציר
בעשורים האחרונים הייצוג של תנועות הושטה במוח של קוף המקק ( )Macaqueנחקר בשיטות אלקטרופיזיולוגיות
במידה רבה .נמצא כי תאי עצב בקליפת המוח התנועתית הראשונית ( )M1מקודדים פרמטרים שונים של תנועה,
ביניהם כיוון תנועה .לאחרונה ,הייצוג של תנועות הושטה נחקר גם במוח האדם באמצעות שיטות שאינן חודרניות,
כולל אלקטרואנצפלוגרפיה ) ,)Electroencephalography, EEGמגנטואנצפלוגרפיה ( Magnetoencephalography,
)MEGותהודה מגנטית תפקודית ( .)fMRIבהמשך למגמה זו ,חקרתי בעבודה זו את כיווניות תנועות ההושטה של
בני אדם באמצעות ,fMRIוניסיתי לגשר על הפער בין אלקטרופיזיולוגיה בקוף המקק לבין הדמיה בבני אדם.
היובל ( )Hubelוויזל ( )Wieselהראו בשנות ה 01של המאה הקודמת שתאי עצב בקליפת המוח הראייתית
הראשונית מאורגנים על פי העדפת האוריינטציה שלהם .כלומר תאי עצב שמעדיפים גירוי ראייתי באוריינטציה
מסוימת על פני אוריינטציות אחרות ,יטו להיות מאורגנים באשכול אחד ,יחד עם תאי עצב שכנים עם העדפה
דומה .מאז ,תאי עצב בקליפת המוח הטמפורלית המדיאלית ( )MTנמצאו מאורגנים על פי העדפתם לכיוון של
תנועה נצפית ,וכן קליפת המוח השמיעתית הראשונית מאורגנת על פי העדפת תדר .ב ,M1ארגון בהתאם
לתפקוד תאי העצב נחקר במידה פחותה ,אך ישנן עדויות לארגון פונקציונאלי מסוים באזור זה על פי העדפתם
לכיוון תנועה של יד הקוף .בבני אדם ,ארגון פונקציונאלי טרם נחקר.
לפיכך ,השתמשתי בפרדיגמה של אירועים מהירים ב ,)fast event related fMRI( fMRIבה נבדקים התבקשו
להזיז סמן באמצעות ג'ויסטיק מהמרכז לכיוון מטרות שונות בפריפריה .המטרה הייתה למצוא העדפה כיוונית
ברמת הווקסל ,בסדר גודל של מספר מילימטרים .מאחר ומקובל לחשוב שכל ווקסל מכיל כמיליון תאי עצב ,נצפה
כי אם תאים אלו מפוזרים בצורה אחידה בווקסל ,עקומת הכיוונון ( )tuning curveתהיה שטוחה ,כלומר הפעילות
של ווקסל כזה תהיה דומה בתגובה לכל אחד מהכיוונים .מצד שני ,העדפה כיוונית ברזולוציה כל כך גסה מאפשרת
הטיה קלה לטובת כיוון אחד על פני האחרים מאחר וארגון באשכולות מוריד את מספר היחידות התפקודיות בתוך
ווקסל .הטיה כזו יכולה להוביל לכיוונון של ווקסל .אכן ,מצאתי ב M1עקומות כיוונון של ווקסלים ,המעידות על
ארגון על פי מאפייני תאי העצב .כיוונון זה הוצג באמצעות מספר כלים אנליטיים )0( :הראיתי בצורה ישירה
שכאשר עקומת הכיוונון מיושרת ל"כיוון המועדף" (הכיוון בו פעילות הווקסל הינה הגבוהה ביותר) ,ישנה ירידה
הדרגתית בפעילות ככל שכיוון התנועה היה רחוק יותר מהכיוון המועדף )2( .השתמשתי באנליזה של דגמי פעילות
( )Multi voxel pattern analysisכדי להראות שדגמי הפעילות המרחביים נמצאים במתאם גבוה עם דגמים של
תגובה לתנועה באותו כיוון .יתרה מזאת ,המתאם בין שני דגמים מרחביים יורד ככל שהמרחק בין הכיוונים עולה.
( )3כאשר חוזרים על תנועה לאותו כיוון פעמיים ברציפות ,ישנה ירידה בפעילות בתנועה החוזרת (Repetition
) .Suppression, RSתופעה זו לא ירדה באופן הדרגתי עם העלייה בהפרש בין הכיוונים ,אך היא מעידה כי סיגנל ה
fMRIאכן רגיש לכיוון תנועה .בנוסף ,השתמשנו במודל שבנינו על מנת להעריך את גודל האשכול .קוטר האשכול
על פי המודל מוערך בכמה מאות מיקרונים ,סדר גודל דומה לזה שהוערך במחקרים אחרים ב M1של הקוף.
I
לאור תוצאות אלו ,נשאלת השאלה לגבי אבחנה בין רכיב תנועת היד לבין הרכיבים הראיתיים של המשימה .האם
ייצוג הכיווניות הוא אכן ייצוג של תנועת היד ,או שמא ישנו ייצוג של רכיבים ראיתיים ,כגון מיקום המטרה או תנועת
הסמן על המסך? בכדי שנוכל לענות על שאלה זו הרצתי ניסוי נוסף עם שני חלקים .החלק הראשון היה בדיוק כמו
בניסוי שתואר לעיל .בחלק השני לעומת זאת ,הסמן סובב ב 54מעלות ביחס לתנועת הג'ויסטיק .סיבוב זה
מאפשר הפרדה בין הרכיבים התנועתיים והראייתיים של המשימה ,מכיוון שכאשר מיקום המטרה דומה בשני
חלקי הניסוי ,תנועת היד שונה ב 54מעלות ,וכאשר התנועה היא לאותו כיוון בשני החלקים ,מיקום המטרה שונה
ב 54מעלות .השתמשתי באותה אנליזה של קורלציות בה השתמשתי בניסוי הקודם ,אלא שכאן חישבתי בנפרד
את המתאם בין מיקומי מטרה שונים ובין כיווני תנועה שונים .המתאם ב M1היה סיגניפיקנטי בשני המקרים.
בעקבות תוצאה זו הרצתי ניסוי נוסף בו נבדקים התבקשו לצפות בסמן נע על המסך לעבר המטרות השונות כך
שהאלמנט הראייתי נשאר כפי שהיה ,אך הסמן לא נשלט על ידי הנבדקים ,והנבדקים התבקשו לא להזיז את
ידיהם במהלך הניסוי .ניסוי זה נבנה על מנת שנוכל לראות אם האלמנטים הראייתיים מספיקים בכדי להביא
לפעילות ב ,M1ואף למתאם גבוה בין תנועות באותו כיוון .אף אחד מהנבדקים בניסוי זה לא הראה כל פעילות ב
M1או מתאם חיובי בין תנועות באותו כיוון .לפיכך ,האלמנטים הראייתיים מקודדים אך ורק כשהם מזווגים לתנועה
של היד ,ומיוצגים ב M1אך ורק כשהם נושאים מידע רלוונטי לתנועה ,כגון מיקום מטרה או פידבק.
שאלה פתוחה נוספת מהניסוי הראשון נוגעת ל .RSהסברה הרווחת היא ש RSמשקף תכונות של תאי עצב
בודדים ,אך למרות שתופעה זו נצפתה בתאי עצב בקליפת המוח הראייתית בתגובה לחזרה על אותו גירוי ראייתי,
אין עדויות ל RSבאזורים תנועתיים .בניסוי הבא ניתחתי נתונים מרישומים אלקטרופיזיולוגיים בקוף מקק וניסיתי
למצוא הבדל בקצב הירי של תאי העצב או בעקומת הכיוונון של אוכלוסיית התאים בין תנועות באותו כיוון כמו
בתנועה הקודמת לבין תנועות בכיוון ההפוך מהתנועה הקודמת .בנוסף ,מאחר והפוטנציאליים המקומיים ( Local
)Field potential, LFPבאזורים ראייתיים גם הראו ,RSוהראו בעבר כי סיגנל ה LFPנמצא בקורלציה עם סיגנל ה
,fMRIציפינו לכל הפחות למצוא בו הבדל בין תנועות חוזרות לבין תנועות הפוכות .התוצאות הראו שאין שום
הבדל בין תנועות חוזרות לבין תנועות הפוכות ,לא בקצב הירי של תאי העצב ,ולא ב .LFPאפילו כשהגבלתי את
בסיס הנתונים לתאי עצב עם העדפה כיוונית ברורה בלבד ,לא נצפה כל הבדל ,לא בכיוון המועדף ולא בכיוון
ההפוך למועדף (הבדל שהיה מעיד על שינוי בעקומת הכוונון) .כדי לענות על השאלה לגבי RSבתאי עצב בצורה
טובה יותר ,ישנו צורך במחקר נוסף ,אך העדר כל אפקט של חזרתיות בתאי העצב יכול להעיד כי ה RSהמוטורי
שנמצא בסיגנל ה fMRIאינו מייצג את הפעילות של תאי העצב ,במידה שנוהגים לחשוב.
II