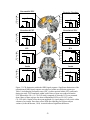
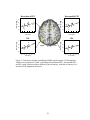
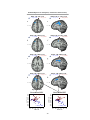
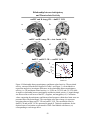
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download NEURAL MECHANISMS SUPPORTING THE LEARNING
Human multitasking wikipedia , lookup
Brain morphometry wikipedia , lookup
Nervous system network models wikipedia , lookup
Human brain wikipedia , lookup
Neuroanatomy wikipedia , lookup
Neurogenomics wikipedia , lookup
Brain Rules wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Embodied language processing wikipedia , lookup
Neural engineering wikipedia , lookup
Time perception wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Perception of infrasound wikipedia , lookup
Neuropsychology wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Biology of depression wikipedia , lookup
Psychophysics wikipedia , lookup
Neuroinformatics wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Neuroplasticity wikipedia , lookup
Aging brain wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Response priming wikipedia , lookup
Evoked potential wikipedia , lookup
Emotion perception wikipedia , lookup
Neurolinguistics wikipedia , lookup
Neuroesthetics wikipedia , lookup
Mental chronometry wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Limbic system wikipedia , lookup
Executive functions wikipedia , lookup
Difference due to memory wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Neuromarketing wikipedia , lookup
Neuroeconomics wikipedia , lookup
Neurophilosophy wikipedia , lookup
Haemodynamic response wikipedia , lookup
History of neuroimaging wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Affective neuroscience wikipedia , lookup
Conditioned place preference wikipedia , lookup
Classical conditioning wikipedia , lookup
Metastability in the brain wikipedia , lookup
NEURAL MECHANISMS SUPPORTING THE LEARNING-RELATED EMOTIONAL RESPONSE TO A THREAT by KIMBERLY H. WOOD DAVID C. KNIGHT, COMMITTEE CHAIR EDWIN W. COOK, III RAJESH K. KANA ADRIENNE C. LAHTI KRISTINA M. VISSCHER A DISSERTATION Submitted to the graduate faculty of The University of Alabama at Birmingham, in partial fulfillment of the requirements for the degree of Doctor of Philosophy BIRMINGHAM, ALABAMA 2013 Copyright by Kimberly H. Wood 2013 NEURAL MECHANISMS SUPPORTING THE LEARNING-RELATED EMOTIONAL RESPONSE TO A THREAT KIMBERLY H. WOOD BEHAVIORAL NEUROSCIENCE ABSTRACT Successful regulation of the emotional response to a threat allows one to react more effectively under threatening conditions. The prefrontal cortex (PFC) and amygdala are key brain regions that mediate the regulation and expression of emotion. We employed Pavlovian fear conditioning to investigate the neural mechanisms that influence the emotional response to a threat. These procedures were designed to investigate conditioned diminution of the unconditioned response (UCR). The specific aims were to better understand the role of associative learning, expectation, controllability, and predictability in modulating UCR expression. This project employed functional magnetic resonance imaging (fMRI) to assess the magnitude of the threat-related response within the PFC, cingulate cortex, inferior parietal lobule, insula, amygdala, and hippocampus. We also investigated the peripheral expression of emotion indexed via skin conductance response (SCR) and startle eye-blink electromyography (EMG) during differential fear conditioning. To assess the effect of expectation of an impending threat, volunteers provided a continuous self-report measure of UCS expectancy throughout the conditioning sessions. We also examined whether individual differences in anxiety level influenced the emotional response to a threat. In general, we observed a relationship between anxiety level and the threat-related neurophysiological response. Conditioned UCR diminution within the neurophysiological response was also observed. More specifically, the threat-related fMRI signal response iii and SCR expression was diminished on predictable vs. unpredictable trials. However, the opposite pattern was observed in the EMG data. An enhanced startle-eyeblink response was observed for predictable compared to unpredictable trials. Further, controllability affected the threat-related fMRI signal response within the ventromedial PFC and hippocampus. The unconditioned SCR elicited by the threat paralleled the fMRI signal response within several brain regions that showed UCR diminution. A negative relationship was observed between UCS expectancy and the threat-related response within several brain regions that showed conditioned UCR diminution. In summary, we observed learning-related changes in the emotional response to a threat within regions of the PFC, amygdala, and hippocampus. The current findings suggest that these brain areas support learning-related processes that modulate the emotional response to a threat. Keywords: fMRI, emotion, fear conditioning, threat response, prefrontal cortex, amygdala iv DEDICATION This dissertation is dedicated to my husband and daughters, Robert, Krista, and Kayli Wood. Thank you for the encouragement and support during this academic journey over the last fourteen years. I could not have done this without you! v ACKNOWLEDGEMENTS Thank you to my mentor Dr. David C. Knight for his guidance and generosity throughout my graduate career. Thanks to my dissertation committee for their time and feedback in the development of this project. I appreciate all past and present members of our lab. Thank you all for the much needed laughs. Special thanks to Josh and Muriah for their contribution to data acquisition and analysis. I would like to thank the Behavioral Neuroscience Directors and past and present students. To Drs. Randich and Amthor, it has been such a privilege to be under your academic advisement and your student. Thank you to the Behavioral Neuroscience students for your advice. Thank you to the Department of Psychology for their administrative and financial support during my graduate training. A special thanks to my husband and daughters, Robert, Krista, and Kayli for their love and encouragement throughout my academic career. Words cannot express how truly thankful I am to have such an amazing family. Thank you to all my family, church family, and friends for your support and encouragement throughout my graduate career. vi TABLE OF CONTENTS Page ABSTRACT ....................................................................................................................... iii DEDICATION .....................................................................................................................v ACKNOWLEDGEMENTS ............................................................................................... vi LIST OF TABLES ........................................................................................................... viii LIST OF FIGURES ........................................................................................................... ix LIST OF ABBREVIATIONS ............................................................................................ xi INTRODUCTION ...............................................................................................................1 Modulation of the UCR ............................................................................................2 PFC-amygdala network ...........................................................................................5 Controllability and predictability ............................................................................6 Anticipation and anxiety ..........................................................................................7 Specific Aims ............................................................................................................9 NEURAL MECHANISMS UNDERLYING THE CONDITIONED DIMINUTION OF THE UNCONDITIONED FEAR RESPONSE..................................12 NEURAL SUBSTRATES UNDERLYING LEARNING-RELATED CHANGES OF THE UNCONDITIONED FEAR RESPONSE .......................................58 CONTROLLABILITY AND PREDICTABILITY DIMINISH THE NEURAL RESPONSE TO A THREAT...................................................................93 SUMMARY .....................................................................................................................124 GENERAL LIST OF REFERENCES .............................................................................128 APPENDIX: IRB APPROVAL FORM..........................................................................134 vii LIST OF TABLES Table Page NEURAL MECHANISMS UNDERLYING THE CONDITIONED DIMINUTION OF THE UNCONDITIONED FEAR RESPONSE 1 Regions showing conditioned diminution of the UCR ................................................46 2 Regions showing change over time .............................................................................47 3 Regional activity varying with trait anxiety.................................................................48 4 Regions showing a relationship between anticipatory and threat-related activity ........................................................................49 NEURAL SUBSTRATES UNDERLYING LEARNING-RELATED CHANGES OF THE UNCONDITIONED FEAR RESPONSE 1 Regions showing conditioned diminution of the UCR ............................................... 85 2 Regions showing potentiation of the UCR ..................................................................86 3 Regions showing change over time .............................................................................87 4 Regions showing a relationship between anticipatory and threat-related activity ........................................................................88 CONTROLLABILITY AND PREDICTABILITY DIMINISH THE NEURAL RESPONSE TO A THREAT 1 Demographics and group characteristics ...................................................................117 2 Regions showing conditioned diminution of the UCR ..............................................118 3 Regional activity varying with state anxiety ..............................................................119 viii LIST OF FIGURES Table Page NEURAL MECHANISMS UNDERLYING THE CONDITIONED DIMINUTION OF THE UNCONDITIONED FEAR RESPONSE 1 Conditioning procedure ...............................................................................................50 2 UCS expectancy and unconditioned SCR....................................................................51 3 UCR diminution within the fMRI signal response ......................................................52 4 Stimulus x trial interaction within the ventromedial PFC ...........................................53 5 Trait anxiety and the unconditioned fMRI signal response .........................................54 6 Relationship between amygdala and unconditioned SCR ...........................................55 7 Relationship between anticipatory and threat-related activity .....................................56 NEURAL SUBSTRATES UNDERLYING LEARNING-RELATED CHANGES OF THE UNCONDITIONED FEAR RESPONSE 1 Conditioned and unconditioned stimuli ...................................................................... 89 2 UCS expectancy and unconditioned SCR....................................................................90 3 UCR diminution within the fMRI signal response ......................................................91 4 Relationship between anticipatory and threat-related activity .............................................92 CONTROLLABILITY AND PREDICTABILITY DIMINISH THE NEURAL RESPONSE TO A THREAT 1 Acquisition phase .......................................................................................................120 2 UCS Expectancy, unconditioned SCR, and EMG response ......................................121 ix 3 Conditioned UCR diminution within the fMRI signal response ...............................122 4 Regions showing predictability x controllability interaction .....................................123 x LIST OF ABBREVIATIONS CS conditioned stimulus CS+ CS paired with the unconditioned stimulus CS− CS presented alone CR conditioned response SCR skin conductance response EMG electromyography UCR unconditioned response UCS unconditioned stimulus xi INTRODUCTION Fear is considered an important defense mechanism due to its evolutionary role in survival (Kim & Jung, 2006; LeDoux, 2003). Consequently, certain environmental stimuli have become innately hardwired over our evolutionary history to induce fear (e.g. loud noises, darkness). However, fear can also be rapidly associated with neutral stimuli, thereby permitting animals to adapt to an ever changing environment (Domjan, 2005; Kim & Jung, 2006; LeDoux, 2003). This adaptation of the fear response has been observed in a wide range of species and response systems using Pavlovian fear conditioning (Davis, 1992; Domjan, 2005; Helmstetter & Bellgowan, 1994; Kim & Jung, 2006). Further, the ability to form associations between a dangerous event and the cues that predict it allows an organism to more effectively minimize the impact of an impending threat. For example, conditioned hypoalgesia, (ie. decreased pain sensitivity) is observed to painful stimuli during Pavlovian conditioning (Bellgowan & Helmstetter, 1996; Helmstetter, 1992). This reduction in the response to a threat is also observed during Pavlovian fear conditioning when the unconditioned response (UCR) is diminished to predictable compared to unpredictable presentations of an unconditioned stimulus (UCS) (Domjan, 2005). From a functional perspective, it is the response to the threat itself (i.e. the UCS) that is the most important component of Pavlovian conditioning (Domjan, 2005; Pavlov, 1927). Further, fear responses in Pavlovian conditioning closely resemble characteristic traits of human anxiety disorders, thus, understanding the biological mechanisms of fear 1 conditioning may elucidate the behavioral and physiological abnormalities of emotion regulation in fear-related disorders (Davis, 1992; Kim & Jung, 2006; LeDoux, 2007; Maren, 2001; Milad et al., 2007). Pavlovian conditioning, often referred to as classical conditioning, is one of the oldest and most straightforward paradigms to study fear-related processes (Domjan, 2005; LeDoux, 1998; Pavlov, 1927). It is considered a model system to investigate the neurobiological mechanisms of learning (Fanselow & Ledoux, 1999; Helmstetter & Bellgowan, 1993; Kim & Jung, 2006; Maren, 2001). During Pavlovian fear conditioning, a neutral conditioned stimulus (CS) is paired with an aversive UCS. The conditioned response (CR) produced by the CS is often used to index fear expression. Traditionally, CR expression is taken as evidence that an association between the CS and UCS has been formed. In contrast, the UCR is often considered an automatic, reaction to the aversive UCS that does not require associative learning. Although CR expression is evidence of adapting to environmental change, there are also associative learning-related changes in the UCR to the threat itself. Further, learning-related changes in the UCR, produced by the UCS, directly impacts survival and therefore may be the most biologically relevant feature of Pavlovian conditioning (Domjan, 2005; Pavlov, 1927). Thus, a better understanding of the neural mechanisms that mediate learning-related changes in the UCR is warranted. Modulation of the UCR Learning-related changes in the UCR have been observed in a variety of response systems. These changes can be exhibited as an increase or decrease in the UCR to the 2 UCS. For example, prior work has shown potentiation of the startle response during fear conditioning (Grillon et al., 1991), while unconditioned skin conductance response (SCR) diminishes as associative learning develops during Pavlovian fear conditioning (Baxter, 1966; Kimmel, 1967; Marcos & Redondo, 1999) . More specifically, the magnitude of the unconditioned SCR decreases as the CS and UCS are repeatedly paired (Baxter, 1966). Although some of this early work could be influenced by habituation of the UCR, other research indicates UCR amplitude is decreased to paired compared to unpaired presentations of the CS and UCS (Kimmel, 1967), and UCR magnitude is smaller to predictable compared to unpredictable UCS presentations (Lykken et al., 1972; Peeke & Grings, 1968). These findings indicate the reduction in UCR amplitude during Pavlovian conditioning cannot be solely explained by a simple non-associative learning process (i.e. habituation). Instead, the findings suggest that presentation of the CS+ (i.e. stimulus that predicts the UCS) modulates UCR expression via associative learning processes (Baxter, 1966; Kimmel, 1967; Knight et al., 2010, 2011; Marcos & Redondo, 1999). This phenomenon is generally referred to as conditioned UCR diminution. UCR diminution has been investigated using differential conditioning procedures that consist of a training session in which one CS is paired with the UCS (CS+) and a second CS is presented alone (CS−), followed by a testing session where both the CS+ and CS− are paired with the UCS (Knight et al., 2011; Marcos & Redondo, 1999; Wood et al., 2012). This work has demonstrated greater UCR diminution when the UCS follows the CS+ compared to when the UCS follows the CS− on test trials (Knight et al., 2011; Marcos & Redondo, 1999; Wood et al., 2012). This prior work indicates that UCR diminution is in part mediated by an associative learning process in which the CS+ gains 3 discriminative control over the UCR (Baxter, 1966; Kimmel, 1967; Knight et al., 2010; Marcos & Redondo, 1999). Others have suggested that conscious expectations modify UCR expression (Dunsmoor et al., 2008; Knight et al., 2010; Rust, 1976). For example, greater UCR diminution has been observed when participants expect a UCS compared to when the UCS is unexpected (Dunsmoor et al., 2008; Knight et al., 2010). Additionally, graded increases in UCS expectancy are paralleled by graded decreases in unconditioned SCR magnitude (Dunsmoor et al., 2008; Knight et al., 2010). These findings suggest that associative learning processes and conscious UCS expectancies modulate the expression of UCRs. Few brain imaging studies have used functional magnetic resonance imaging (fMRI) to investigate the neural substrates that support conditioned UCR diminution. In this previous research, UCR diminution has been observed within the fMRI signal response of the dorsolateral prefrontal cortex (dlPFC), anterior cingulate cortex (ACC), and amygdala (Dunsmoor et al., 2008; Wood et al., 2012). Further, these studies also found that UCS expectancy varied with the amplitude of the fMRI signal response within several brain regions that showed learning-related changes (Dunsmoor et al., 2008; Wood et al., 2012). For example, UCS expectancy increased during the CS and the amplitude of the fMRI signal response to the UCS within these brain regions decreased (Dunsmoor et al., 2008). Similar findings have been observed within ventromedial (vmPFC) and dorsomedial (dmPFC) prefrontal cortices as well (Knight et al., 2010; Wood et al., 2012). These studies also found that when the magnitude of the unconditioned fMRI signal response within the dmPFC, dlPFC, and insula increased, a larger autonomic response (e.g. SCR) was produced. Similar to the learning-related changes observed within the 4 fMRI signal response, as UCS expectancy increased the magnitude of unconditioned SCRs decreased (Knight et al., 2010). These findings suggest that diminution of the behavioral UCR is, at least, partly mediated by via prefrontal brain regions that support expectancy-related processes (Dunsmoor et al., 2008; Knight et al., 2010; Wood et al., 2012). PFC-amygdala network Prior work has implicated interconnected dorsal and ventral neural systems that independently process the properties of stimuli (Kahnt et al., 2011; Morgane et al., 2005), including emotionally valenced stimuli (Viinikainen et al., 2010). The ventral system consists of ventral regions of the PFC such as the orbitofrontal cortex (OFC) and vmPFC, as well as insular cortex, and subgenual and pregenual regions of the ACC (Phillips et al., 2003). The ventral system also includes subcortical brain structures such as the amygdala, ventral striatum, thalamus, and hypothalamus (Phillips et al., 2003). The ventral system is essential in the assessment of the emotional significance of stimuli within the environment and the production of the emotional response (Phillips et al., 2003). The dorsal system consists of dmPFC, dlPFC, dorsal and caudal regions of the ACC, and the hippocampus (Phillips et al., 2003). The dorsal system is responsible for integrating cognitive processes and executive planning functions that can be influenced by emotional input (Büchel et al., 1998; Phillips et al., 2003). The dorsal and ventral systems also differ in the type of responses produced. The dorsal system is important for effortful cognitive regulation of emotional responses, whereas the ventral system contributes to the generation of automatic responses (Phillips et al., 2003). However, the role of these 5 dorsal and ventral systems in the modulation of the UCR is currently unknown. These dorsal and ventral neural systems also play an important role in human fear conditioning (Phillips et al., 2003). During fear acquisition, ACC activity increases as the CS and UCS pairing rate increases (Dunsmoor et al., 2007). The amygdala also plays a key role in forming the CS and UCS association (Helmstetter, 1992; LeDoux, 2007), as well as detecting changes in the relationship between the CS and UCS (Knight et al., 2004). Further, the PFC modulates subcortical brain regions, like the amygdala, which controls expression of the emotional response during fear conditioning (Delgado et al., 2008; Knight et al., 2005). For example, prior work has demonstrated similarities in the pattern of activity within the fMRI signal response of the dmPFC, dlPFC, and vmPFC that correspond with SCR expression during fear conditioning (Knight et al., 2010). These findings demonstrate learning-related changes within the dorsal and ventral neural systems during human Pavlovian fear conditioning. However, it remains unclear as to how these brain areas specifically contribute to conditioned diminution of the UCR. Controllability and predictability Prior work suggests that top-down mechanisms support contingency learning and emotional regulation (Dunsmoor et al., 2008; Kim & Jung, 2006; Knight et al., 2010), and that both cortical and subcortical brain regions process aversive stimuli (Delgado et al., 2008; Milad et al., 2004). Cortical and subcortical brain regions (e.g. dlPFC, ACC, insula, and amygdala) also respond differentially based on the ability to control a UCS in both humans (Salomons et al., 2004, 2007; Wiech et al., 2006) and animals (Amat et al., 2005; Baratta et al., 2007, 2008; Maier et al., 2006). Prior animal research suggests that 6 the medial PFC (mPFC) inhibits the response of subcortical brain areas (e.g. the amygdala) when a UCS is controllable (Baratta et al., 2007; Foa et al., 1992; Maier et al., 2006). More importantly, exposure to a controllable UCS alters the impact of that UCS when encountered in the future (Amat et al., 2005; Baratta et al., 2007; Maier et al., 2006). For example, exposure to a controllable UCS interferes with subsequent fear conditioning, whereas exposure to an uncontrollable UCS potentiates the conditioned fear-response (Baratta et al., 2007; Maier et al., 2006). Similar investigations of controllability have been conducted in neuroimaging studies using fMRI. However, in this prior work participants received trials of both controllable and uncontrollable presentations of a painful stimulus (Salomons et al., 2004, 2007; Wiech et al., 2006). These studies found greater activation within dlPFC, ACC, insula, and the amygdala in response to an uncontrollable UCS compared to a controllable UCS. Additionally, increased activation within dlPFC, ACC, and vmPFC has been observed in anticipation of an uncontrollable UCS compared to a controllable UCS (Salomons et al., 2007). However, these studies did not include unpredictable presentations of controllable and uncontrollable aversive stimuli (Salomons et al., 2004, 2007; Wiech et al., 2006). Therefore, it remains unclear as to how the ability to control a UCS during Pavlovian fear conditioning may influence the PFC-amygdala circuit and effect UCR diminution. Anticipation and anxiety A number of neuroimaging studies have investigated anticipation of aversive events using a variety of paradigms (Critchley et al., 2001; Herwig et al., 2007; Nitschke et al., 2006; Volz et al., 2003). These studies have suggested the amygdala, dlPFC, insula, and ACC support anticipatory processes (Schienle et al., 2010). For example, the ACC 7 and amygdala respond to cues that predict aversive stimuli during fear conditioning (Büchel et al., 1998; Davis & Whalen, 2001; Knight et al., 1999; Nitschke et al., 2006; Phillips et al., 2003) and show increased activity when participants are not certain of the outcome (Critchley et al., 2001; Davis & Whalen, 2001; Dunsmoor et al., 2007; Volz et al., 2003). Anticipation of a possible threat occurring in the future is a key feature of anxiety-related disorders (Davis et al., 2009; Grillon, 2002; Nitschke et al., 2009, 2006). Therefore, human neuroimaging studies have also investigated the PFC-amygdala circuit as a function of anxiety. For example, during cued and contextual fear conditioning, recruitment and sustained activation of vmPFC was observed among participants with low trait anxiety, but not participants with high trait anxiety (Indovina et al., 2011). Furthermore, vmPFC activity was inversely related to fear conditioned SCRs for participants with low trait anxiety, but not those with high trait anxiety (Indovina et al., 2011). Prior work has also shown that anxiety level influences ACC activity that is required to attend and respond to threatening stimuli (Klumpp et al., 2011). Specifically, high trait anxiety was associated with decreased activation within the ACC (Klumpp et al., 2011; Sehlmeyer et al., 2011) in conjunction with an exaggerated amygdala response (Sehlmeyer et al., 2011). Taken together, these findings support previous research that suggests that anxiety level influences top-down regulatory control of the fear response (Kim et al., 2011; Milad et al., 2009; Rauch et al., 2006). Given that anxiety level affects anticipatory reactivity and the response to aversive stimuli, differences in the level of anxiety individuals experience may also influence UCR diminution. 8 Specific Aims In summary, prior Pavlovian conditioning research suggests that regions of the PFC modulate the emotional response controlled by the amygdala (Delgado et al., 2008; Kim & Jung, 2006; Milad et al., 2006, 2009; Rauch et al., 2006). Therefore, PFC modulation of the amygdala may also mediate learning-related changes in UCR expression during fear conditioning (Dunsmoor et al., 2008; Knight et al., 2010). However, there remains a critical gap in our understanding of the mechanisms that contribute to conditioned diminution of the UCR. Specifically, partially independent associative learning and expectancy-related processes appear to influence UCR diminution (Kimmel, 1967; Marcos & Redondo, 1999). However, the neural circuitry that supports these processes remains unclear. This project used a multi-modal approach to investigate learning-related changes in the emotional response evoked by a threat. We combined neurophysiological, behavioral, and self-assessment measures to examine UCR diminution during Pavlovian fear conditioning. These measures included a continuous self-report of conscious UCS expectancy, unconditioned SCR, and startle eye-blink electromyography (EMG) response produced by the UCS. By incorporating these indices, the effect of UCS expectation on the magnitude of brain (i.e. fMRI) and autonomic responses (i.e. SCR and EMG) to the UCS can be observed. Additionally, self-assessment measures (i.e. anxiety level) were used to evaluate processes that affect UCR diminution. The primary objective of this project was to attain a better understanding of the neurophysiological mechanisms that effect conditioned UCR diminution using Pavlovian fear conditioning. These studies investigated the role that associative learning, UCS expectancy, controllability, predictability, and anxiety level play in 9 the conditioned diminution of the UCR. The central hypothesis was that the magnitude of unconditioned dlPFC, dmPFC, vmPFC, and amygdala response is mediated by associative learning, expectation, controllability, and predictability of the UCS. We also expected that anxiety level would influence the magnitude of the threat-related neurophysiological response. Manuscript 1 – In this study, we used fMRI to compare threat-related brain activity and SCR expression to predictable and unpredictable presentations of the UCS using CSs that were easy to discriminate. This differential conditioning procedure was followed by a test phase that consisted of UCS presentations that followed the CS+ and CS−, as well as presentations of the UCS alone to assess associative learning and expectancy-related processes that support conditioned UCR diminution. We expected to observe learning-related changes in the unconditioned fMRI signal response within regions of the PFC and amygdala that influence the emotional response (indexed via SCR). We also assessed the relationship between threat-related activity within brain regions that showed UCR diminution and individual differences in trait anxiety. Manuscript 2 – The purpose of this study, was to better understand the neural substrates that support associative learning processes that influence UCR expression in the absence of differential UCS expectancies. The differential conditioning procedure was also followed by a test phase that consisted of UCS presentations that followed the CS+ and CS−, as well as presentations of the UCS alone. However, this paradigm used CSs that were difficult to discriminate to assess associative learning independent of 10 expectancy-related processes that influence the threat-related fMRI signal and SCR expression. We hypothesized UCR diminution would be observed independent of conscious UCS expectancies. Further, we expected to observe learning-related changes within the PFC-amygdala circuit that mediate the emotional response (indexed via SCR). Manuscript 3 – In this study, we used predictable and unpredictable presentations of the UCS to investigate conditioned UCR diminution. We also assessed the effect of UCS controllability on UCR diminution. For this study, there were two groups that consisted of yoked pairs where one group (Controllable Condition) could terminate the UCS, and the other group (Uncontrollable Condition) could not terminate the UCS. We also assessed the influence of UCS expectancy and state anxiety in the modulation of the threat-related fMRI signal, SCR production, and EMG response. We hypothesized that controllability and predictability would influence the magnitude of the unconditioned neurophysiological response. Further, we expected to observe a relationship between anxiety level and the magnitude of the threat-related emotional response produced within the PFC-amygdala circuit, as well as emotional expression (indexed via SCR and EMG). 11 12 NEURAL MECHANISMS UNDERLYING THE CONDITIONED DIMINUTION OF THE UNCONDITIONED FEAR RESPONSE by KIMBERLY H. WOOD, LAWRENCE W. VER HOEF, AND DAVID C. KNIGHT NeuroImage 60, 787–799 Copyright 2012 by Elsevier Used by permission Format adapted and errata corrected for dissertation Abstract Recognizing cues that predict an aversive event allows one to react more effectively under threatening conditions, and minimizes the reaction to the threat itself. This is demonstrated during Pavlovian fear conditioning when the unconditioned response (UCR) to a predictable unconditioned stimulus (UCS) is diminished compared to the UCR to an unpredictable UCS. The present study investigated the functional magnetic resonance imaging (fMRI) signal response associated with Pavlovian conditioned UCR diminution to better understand the relationship between individual differences in behavior and the neural mechanisms of the threat-related emotional response. Healthy volunteers participated in a fear conditioning study in which trait anxiety, skin conductance response (SCR), UCS expectancy, and the fMRI signal were assessed. During acquisition trials, a tone (CS+) was paired with a white noise UCS and a second tone (CS−) was presented without the UCS. Test trials consisted of the CS+ paired with the UCS, CS− paired with the UCS, and presentations of the UCS alone to assess conditioned UCR diminution. UCR diminution was observed within the dorsolateral PFC, dorsomedial PFC, cingulate cortex, inferior parietal lobule (IPL), anterior insula, and amygdala. The threat-related activity within the dorsolateral PFC, dorsomedial PFC, posterior cingulate cortex, and inferior parietal lobule varied with individual differences in trait anxiety. In addition, anticipatory (i.e. CS elicited) activity within the PFC showed an inverse relationship with threat-related (i.e. UCS elicited) activity within the PFC, IPL, and amygdala. Further, the emotional response (indexed via SCR) elicited by the threat was closely linked to amygdala activity. These findings are consistent with the view that 13 the amygdala and PFC support learning-related processes that influence the emotional response evoked by a threat. Key words: fMRI, learning, conditioning, unconditioned response, amygdala, prefrontal cortex, emotion, fear, anxiety, skin conductance 14 Introduction The ability to identify and successfully respond to dangers within one’s environment is critical to survival. Learning the relationship between a threat and the cues that predict it allows one to more effectively react under threatening conditions and avoid or minimize harm (Domjan, 2005; Franchina, 1969; Helmstetter & Bellgowan, 1993; Kamin, 1954; Kim & Jung, 2006). Learning the cues that predict an aversive event results in the production of a fear conditioned response (CR) in anticipation of the threat. It is this CR that is often the primary focus of conditioning studies. Although the CR produced by the warning cue is often used as evidence of learning, there are also learning-related modifications to the unconditioned response (UCR) produced by the threat itself. From a functional perspective, it is important to understand these innate UCRs to naturally occurring threats due to their biological relevance for survival (Domjan, 2005). During Pavlovian conditioning a conditioned stimulus (CS) is paired with an aversive unconditioned stimulus (UCS). Associative learning is apparent when the CS generates a CR. The UCR elicited by the UCS is typically considered a reflexive, unlearned response. However, prior work has demonstrated learning-related changes in the UCR. For example, behavioral studies have demonstrated a reduction in UCR amplitude to predictable compared to unpredictable UCS presentations (Baxter, 1966; Knight et al., 2011; Lykken et al., 1972; Lykken & Tellegan, 1972; Peeke & Grings, 1968). More specifically, UCR amplitude is decreased when the UCS follows a CS presentation compared to presentations of the UCS alone (Baxter, 1966; Kimmel, 1967). This phenomenon is generally referred to as UCR diminution. Prior studies indicate that 15 UCR diminution is partly mediated by an associative learning process (Baxter, 1966; Kimmel, 1967; Knight et al., 2011; Marcos & Redondo, 1999; Redondo & Marcos, 2002). In addition, UCR diminution appears to be modulated by conscious UCS expectancies, such that as expectation of the UCS increases, UCR amplitude decreases (Dunsmoor et al., 2008; Knight et al., 2010, 2011; Rust, 1976; Sarinopoulos et al., 2010). Diminution of the UCR during Pavlovian conditioning is consistent with formal learning theory that suggests; 1) learning occurs when there is a discrepancy between an expectation and the outcome, 2) the CS gains discriminative control over the UCR to the UCS during conditioning, and 3) that the UCR is diminished by a predictable compared to unpredictable UCS (Rescorla, 1988; Rescorla & Wagner, 1972; Wagner & Brandon, 1989). For example, prior work has demonstrated a decrease in the magnitude of brain activation once the relationship between a CS and UCS has been learned and is predictable (Casey et al., 2000; Dunsmoor et al., 2008; Fletcher et al., 2001; Knight et al., 2010; Linnman et al., 2011). In contrast, brain activity increases when an outcome violates expectancies (Casey et al., 2000; Fletcher et al., 2001; Knight et al., 2010). Only a few brain imaging studies have investigated the neural substrates that mediate UCR diminution. Dunsmoor et al. (2008) explored UCR diminution during Pavlovian fear conditioning with functional magnetic resonance imaging (fMRI), and demonstrated conditioned diminution of the UCR within the dorsolateral prefrontal cortex (PFC), dorsomedial PFC (including the anterior cingulate cortex), and amygdala. Similar findings have been observed within the ventromedial PFC, dorsomedial PFC, insula, and inferior parietal lobule (IPL) as well (Knight et al., 2010). Further, these studies found that as UCS expectancy increased (during the CS), the amplitude of the 16 fMRI signal response to the UCS decreased within many of these brain regions (Dunsmoor et al., 2008; Knight et al., 2010). Related work suggests that anticipatory activity within the dorsomedial PFC modulates insula and amygdala responses to aversive stimuli (Sarinopoulos et al., 2010). Further, this work suggests that UCS probability influences the magnitude of brain activation produced by an aversive UCS (Dunsmoor et al., 2008; Sarinopoulos et al., 2010). Taken together, these studies suggest that diminution of the behavioral UCR is at least partly mediated by UCS expectancies that are supported by prefrontal brain regions (Dunsmoor et al., 2008; Knight et al., 2010; Sarinopoulos et al., 2010). In addition, the unconditioned fMRI signal response within the dorsomedial PFC, dorsolateral PFC, and insula appears to vary with the magnitude of unconditioned SCRs (Knight et al., 2010). These findings suggest these brain regions modulate the expression of threat-related emotional responses. A number of neuroimaging studies have investigated brain activity during the anticipation of aversive events (Büchel et al., 1998; Critchley et al., 2001; Knight et al., 1999; LaBar et al., 1998; Nitschke et al., 2006). This work indicates that the PFC, insula, and amygdala respond in anticipation of aversive stimuli and show increased activity when participants have uncertain expectations (Critchley et al., 2001; Davis & Whalen, 2001; Dunsmoor et al., 2007; Volz et al., 2003). For instance, anticipatory responses within the insula and dorsolateral PFC are larger when participants are uncertain of whether a UCS will be presented (Dunsmoor et al., 2007). The medial PFC also appears to be important during unpredictable situations. This region may be essential for the development of coping strategies and response selection when conditions are unpredictable (Schienle et al., 2010). Consistent with this view, previous research has 17 demonstrated the importance of the PFC in emotion-related behavior (Delgado et al., 2008; Ochsner et al., 2002). Prior work also suggests that individual differences in anxiety levels influence activity within the neural circuitry that mediates fear-related processes (Klumpp et al., 2011). More specifically, conditioning research indicates that anxiety levels affect anticipatory responses within the amygdala and PFC (Indovina et al., 2011). Others have demonstrated that activity within brain regions that support regulatory control processes is influenced by the level of trait anxiety (Basten et al., 2011; Sehlmeyer et al., 2011). For example, an inverse relationship has been observed between trait anxiety and dorsomedial PFC activity during fear extinction (Sehlmeyer et al., 2011). Other research has demonstrated differences in the functional connectivity of the dorsomedial PFC and amygdala in high compared to low anxious individuals (Kim et al., 2011). Taken together, these findings suggest that individual differences in trait anxiety may modulate activity within the brain regions that support UCR diminution, and in turn, may effect the peripheral expression of the emotional response. Prior work suggests that interconnected dorsal and ventral brain systems process distinct aspects of emotional stimuli (Kahnt et al., 2011; Morgane et al., 2005; Viinikainen et al., 2010). A dorsal system that consists of dorsomedial and dorsolateral PFC appears to be responsible for integrating executive planning functions and higher level cognitive processes that can be influenced by emotional input (Phillips et al., 2003). Further, this dorsal system appears to be important for the effortful cognitive regulation of the emotional response (Delgado et al., 2008; Herwig et al., 2007; Ochsner et al., 2002). A ventral system that includes the amygdala and insula appears to play an 18 important role in assessing the significance of stimuli and producing the emotional response (Cheng et al., 2003, 2006, 2007; Knight et al., 2005; Phillips et al., 2003). However, the role of these dorsal and ventral systems in the threat-related emotional response is in need of further study. The present study investigated trait anxiety, UCS expectancy, unconditioned SCR, and fMRI signal responses during Pavlovian conditioned UCR diminution to better understand the processes that influence learning-related changes in the emotional response to a threat. Prior research indicates that UCR diminution is mediated by associative learning (Baxter, 1966; Kimmel, 1967; Knight et al., 2010; Marcos & Redondo, 1999; Redondo & Marcos, 2002) and conscious expectations of the UCS (Dunsmoor et al., 2008; Knight et al., 2010, 2011; Rust, 1976). Based on previous research, we expected a decrease in the unconditioned fMRI signal to develop with associative learning and expectations of the UCS (Dunsmoor et al., 2008; Knight et al., 2010; Sarinopoulos et al., 2010). Given the importance of the amygdala and dorsolateral, dorsomedial, and ventromedial PFC in emotion (Delgado et al., 2008; Dunsmoor et al., 2008; Kim & Jung, 2006; Ochsner et al., 2002; Sarinopoulos et al., 2010), we hypothesized that these brain regions would show UCR diminution during Pavlovian conditioning. Further, we expected activity within these brain regions to vary with individual differences in UCS expectancy and trait anxiety. In turn, we expected amygdala activity to vary with the learning-related modulation of unconditioned SCRs (Cheng et al., 2003, 2006, 2007; Knight et al., 2005). 19 Materials and Methods Participants: Twenty-four healthy right-handed volunteers participated in this study and were included in the behavioral data analyses [12 male, 12 female; age = 20.83 ± 0.64 years (mean ± SEM); range = 19-33 years]. Three participants were excluded from the fMRI analysis due to poor data quality leaving a total of twenty-one participants (12 male, 9 female; age = 20.81 ± 0.71 years; range = 19-33 years). All subjects provided written informed consent in compliance with the University of Alabama at Birmingham Institutional Review Board. State-Trait Anxiety Inventory: Participants completed the State-Trait Anxiety Inventory (STAI; Form Y) for Adults (Spielberger, 1983) prior to the conditioning session. The STAI consists of self-assessment scales that measure state and trait anxiety in terms of negative affect in general (Grös et al., 2007). Scores on the state scale reflect current anxiety levels, while trait anxiety scores reflect a relatively long-term predisposition for anxiety (Spielberger, 1983). Conditioned and unconditioned stimuli: Participants were exposed to a differential fear conditioning procedure in which the conditioned and unconditioned stimuli were presented through MR-compatible pneumatic headphones. Two tones (700 & 1300 Hz; 10s duration; 20s ITI) served as the CSs and a loud (100db) white-noise served as the UCS (0.5s duration). The UCS coterminated with one tone (CS+), whereas the second tone was presented alone (CS−) during acquisition trials. A total of thirty-two trials of each CS were presented over four 590s acquisition blocks (8 trials of each CS were 20 presented in each block). Additionally, each acquisition block contained one set of 3 test trials that consisted of UCS presentations that coterminated with the CS+ (CS+UCS) and CS− (CS−UCS), as well as presentations of the UCS alone (Figure 1). Thus, acquisition blocks consisted of 19 trials (8 CS+ & 8 CS− acquisition trials, as well as 1 CS+UCS, 1 CS−UCS, & 1 UCS alone test trial). Test trials during the acquisition blocks were presented on trials 17-19 of blocks 1 and 2; trials 13-15 of block 3; and trials 9-11 of block 4. The final acquisition block was followed by a 920s block of 30 test trials (10 CS+UCS trials, 10 CS−UCS trials, 10 UCS alone trials). In total, there were 14 test trials for each stimulus (4 from the acquisition blocks, 10 from the test block). We anticipated that the UCS expectancy ratings to these stimuli would rapidly increase once the test block began. Therefore, test trials were included during acquisition blocks in an effort to maintain differential UCS expectancies (i.e. CS+ vs. CS−) for a greater number of trials. The 14 test trials were grouped into the first 7 test trials (Early test trials) and the last 7 test trials (Late test trials) for further analysis. The test trials from the acquisition blocks were included in this analysis and binned in this manner to increase the number of test trials for this contrast, and to reflect the learning-related changes observed in UCS expectancy that developed during the study. The stimuli were counterbalanced and presented in a pseudorandom order such that no more than two trials of the same stimulus were consecutively presented. UCS expectancy: UCS expectancy was used to measure expectation of the UCS and assess whether the relationship between the CS and UCS had been learned. Using Presentation software (Neurobehavioral Systems, Inc.; Albany, CA), a UCS expectancy 21 rating scale was presented on an IFIS-SA LCD (Invivo Corp.; Gainesville, FL) video screen located above the subject's head and viewed through a mirror attached to the RF coil. An MRI compatible joystick (Current Designs; Philidelphia, PA) was used to monitor subjects’ expectancy of receiving the UCS. The joystick controlled a rating bar which was presented throughout the conditioning session on the video screen. Subjects were instructed to rate their UCS expectancy on a moment-by-moment basis using a continuous scale from 0 to 100 (0 = certain the UCS would not be presented, 50 = uncertain whether the UCS would be presented, 100 = certain the UCS would be presented) to reflect their current UCS expectancy. UCS expectancy was calculated as the average response (1s sample) at UCS onset. Additional details on this methodology have been published previously (Knight & Wood, 2011). Skin conductance response: An MRI compatible physiological monitoring system (Biopac Systems; Goleta, CA) was used to collect SCR data. SCR was sampled (2,000Hz) with a pair of disposable radio-translucent electrodes (1cm diameter, Biopac Systems; Goleta, CA) from the distal phalanx of the middle and ring fingers of the nondominant hand. SCR data were processed using Biopac AcqKnowledge 3.9 software. A 1Hz low pass digital filter was applied and SCR data were resampled at 125Hz. Unconditioned SCRs were calculated as the maximum SCR during the 10s following the UCS presentation as compared to baseline (average SCR during 5s prior to CS onset). Functional MRI: Structural and functional imaging was completed on a 3 Tesla Siemens Allegra scanner. High-resolution anatomical images (MPRAGE) were obtained in the 22 sagittal plane using a T1 weighted series (TR=2300ms, TE=3.9ms, flip angle=12⁰, FOV=25.6cm, matrix=256 x 256, slice thickness=1mm, 0.5mm gap) to serve as an anatomical reference. Blood oxygen level dependent fMRI of the entire brain was conducted using a gradient-echo echoplanar pulse sequence in an oblique-axial orientation (TR=2000ms, TE=30ms, flip angle=70º, FOV=24cm, matrix=64 x 64, slice thickness=4mm, no gap) during each block of stimulus presentations. Functional image processing was performed with the Analysis of Functional NeuroImages (AFNI) software package (Cox, 1996). Echo-planar time series data were corrected for slice timing offset, motion corrected, concatenated, reregistered to the fifth volume of the first imaging block, and spatially blurred using a 4mm full-width-at-half-maximum Gaussian filter. Functional MRI data were analyzed at the individual subject level using the input from all stimuli in a multiple linear regression using a gamma variate hemodynamic response function. Regressors to account for brain activity not related to the UCR on test trials included reference waveforms for the CS+ and CS− during acquisition, UCS during acquisition, CS+ and CS− on test trials, joystick movement, and head motion parameters. On average, less than one millimeter of movement occurred during the scanning session (0.76 ± 0.07). The regressors of interest for this study modeled the unconditioned fMRI signal response to UCS presentations during each type of test trial (i.e. CS+UCS, CS−UCS, and the UCS alone). Separate reference waveforms were used for Early and Late test trials in this analysis. Percent signal change on test trials was used as an index of the magnitude of the unconditioned fMRI signal response produced by the UCS. Functional maps reflecting percent signal change were converted to the Talairach and Tournoux stereotaxic coordinate system for group analyses (Talairach & Tournoux, 23 1988). Based on prior work (Dunsmoor et al., 2008; Knight et al., 2010), group level analyses were restricted using an anatomical mask, to the PFC, cingulate cortex, IPL, insula, amygdala, and hippocampus to reduce the number of voxel-wise comparisons. We conducted a repeated-measures ANOVA to test for a main effect of stimulus (CS+UCS, CS−UCS, and UCS alone) and trial (Early vs. Late test trials), as well as a stimulus x trial interaction. A voxel-wise threshold of p< 0.05 (corrected) was employed by using an uncorrected threshold of p< 0.007 and a cluster volume larger than 620mm3 (11 voxels of 3.75 x 3.75 x 4.00mm dimension). These threshold criteria were used to correct for multiple comparisons. These threshold criteria were based on Monte Carlo simulations that were used to reject smaller clusters of activation produced by chance alone (false positives) (Forman et al., 1995; Saad et al., 2006). The Monte Carlo simulation program (AlphaSim) (Cox et al., 1996; Saad et al., 2006) considers multiple factors to determine the combination of cluster volume and voxel-wise uncorrected p threshold. The simulations are based on: (1) the volume of tissue being studied (restricted to brain regions within our anatomical mask in accordance with our a priori hypotheses); (2) voxel size (using the 3.75 x 3.75 x 4.00mm dimensions in which the voxels were originally acquired); (3) spatial smoothness of the data; and (4) the alpha level (Saad et al., 2006). Given our a priori hypotheses and the relatively small volume of the amygdala, we used a voxel-wise threshold of p< 0.007 and a cluster volume larger than 170mm3 (3 voxels of 3.75 x 3.75 x 4.00mm dimension) for this area. Monte Carlo simulations indicated that the cluster size and p< 0.007 threshold criteria result in a FWE corrected significance threshold of p< 0.05. Follow-up t-test comparisons were conducted in SPSS 24 on the mean percent signal change activation passing the significance threshold (p< 0.05 corrected) for the ANOVA. Two different analysis procedures (i.e. correlation and multiple linear regression) were completed to investigate the relationship between our behavioral measures (i.e. trait anxiety, UCS expectancy, and SCR) and the fMRI signal from brain regions that demonstrated UCR diminution in the ANOVA (i.e. functional regions of interest; ROI). The correlation analysis compared the mean percent signal change from all voxels within an ROI to behavioral measures (p< 0.05 Bonferroni corrected). Separate correlation analyses were completed for trait anxiety scale scores, mean UCS expectancy ratings, and mean unconditioned SCR amplitude to determine whether these behavioral measures varied with ROI activity. In addition, a voxel-wise multiple linear regression analysis was conducted to compare these behavioral measures to the unconditioned fMRI signal response within the ROI. Thus, the regression analysis was also limited to the functional ROI identified in the ANOVA. The linear model included participant’s trait anxiety scale score, mean unconditioned SCR amplitude, and mean UCS expectancy rating during each type of test trial (i.e. CS+UCS, CS−UCS, and UCS alone) to assess the relationship between these behavioral measures and the unconditioned fMRI signal response. Regressors that coded for stimulus type were also included in this analysis to determine whether the behavioral measures explained unique variance in the data. One participant was excluded from the regression analysis because the trait anxiety questionnaire was not completed. AlphaSim (Cox et al., 1996; Saad et al., 2006) was used to conduct Monte Carlo simulations limited to the amygdala and functional ROI from our repeatedmeasures ANOVA that demonstrated a main effect of stimulus or stimulus x trial 25 interaction. A voxel-wise threshold of p< 0.007 and a cluster volume larger than 394mm3 (7 voxels of 3.75 x 3.75 x 4.00mm dimension) was employed, resulting in a FWE corrected significance threshold of p< 0.05. Although the correlation and multiple linear regression analyses (described above) used to compare behavioral measures (i.e. trait anxiety, UCS expectancy, and SCR) to the fMRI signal within the function ROI were similar, there were important differences between the two analyses. First, separate correlation analyses were completed for trait anxiety, UCS expectancy, and unconditioned SCR to determine whether these behavioral measures varied with ROI activity. However, the use of separate analyses cannot determine whether the observed brain-behavior relationships are independent. Therefore, the multiple linear regression analysis was completed using a linear model that included regressors for trait anxiety, UCS expectancy, unconditioned SCR, and stimulus type to determine whether these behavioral measures explained unique variance in ROI activity (e.g. independent of stimulus type). Second, the correlation analysis compared each behavioral measure to the average signal from all voxels within a ROI, whereas the regression analysis was completed on a voxel-wise basis (restricted to each ROI). Thus the correlation analyses assessed activity within an ROI as a whole, while the regression analysis evaluated activity within an ROI on a voxel-wise basis. Prior work suggests that PFC activity regulates the emotional response to aversive stimuli (Delgado et al., 2008; Sarinopoulos et al., 2010). Therefore, we completed an additional voxel-wise multiple regression analysis to identify PFC areas in which anticipatory activity varied with the unconditioned fMRI signal response obtained from the amygdala and functional ROI from our ANOVA. This analysis was restricted to 26 CS+UCS and CS−UCS trials because a CS was not presented during UCS alone trials to elicit an anticipatory response. This analysis included a regressor representing trial type (CS+UCS & CS−UCS), a regressor for the amplitude of the unconditioned fMRI signal response from our functional ROI, and a regressor for the interaction of trial type and unconditioned fMRI signal response amplitude. This analysis was restricted to the PFC and cingulate using an anatomical mask. As indicated by Monte Carlo simulations, a voxel-wise threshold of p< 0.007 and a cluster volume larger than 620mm3 (11 voxels of 3.75 x 3.75 x 4.00mm dimension) was employed resulting in a FWE corrected significance threshold of p< 0.05. Results UCS expectancy: Repeated measures ANOVA revealed significant differences in UCS expectancy during test trials. Results showed a main effect for stimulus type (F[1,23] = 58.41, p< 0.05), a main effect for trial (F[1,23] = 63.43, p< 0.05), and a stimulus by trial interaction (F[1,23] = 38.90, p< 0.05). UCS expectancy was greater during Early test trials on CS+UCS presentations than on CS−UCS (t[23] = 6.74, p< 0.05) and UCS alone trials (t[23] = 7.55, p< 0.05). UCS expectancy did not differ for CS−UCS and UCS alone presentations during the Early test trials (t[23] <1.00). On Late test trials however, UCS expectancy to the CS−UCS was greater than expectancy for the UCS alone (t[23] = 4.40, p< 0.05). UCS expectancy to the CS+UCS remained greater than to the UCS alone (t[23] = 6.45, p< 0.05) and CS−UCS (t[23] = 2.01, p< 0.05) on Late test trials (Figure 2a). Skin conductance response: Repeated measures ANOVA also revealed significant differences in unconditioned SCR expression during test trials. There was a main effect 27 for stimulus type (F[1,23] = 4.77, p< 0.05). However, there was no trial effect (F< 1.00) or stimulus by trial interaction (F< 1.00). T-test comparisons revealed a significantly diminished unconditioned SCR for CS+UCS trials (mean ± SEM [adjusted for between subject variance (Loftus & Masson, 1994)]: 0.07 ± 0.02) as compared to CS−UCS trials (0.14 ± 0.02; t[23] = -1.77, p< 0.05) and UCS alone trials (0.11 ± 0.01; t[23] = -2.18, p< 0.05). There was not a significant difference in unconditioned SCR between the CS−UCS and UCS alone (t < 1.00) (Figure 2b). Functional MRI: The fMRI data analysis indicated that several brain regions showed diminution of the unconditioned fMRI signal response (Table 1; Figures 3-4). UCR diminution was observed within the dorsolateral PFC, dorsomedial PFC, anterior insula, IPL, and posterior cingulate cortex (PCC). In each of these regions, the unconditioned fMRI signal response demonstrated a main effect for stimulus type (F[20] > 5.63; p< 0.05 corrected). A main effect for trial (i.e. Early vs. Late test trials) was observed within the dorsolateral PFC, dorsomedial PFC, PCC, and anterior insula (Table 2). A stimulus x trial interaction was observed within ventromedial PFC (Talairach coordinates: 12, 49, 14; volume: 656mm3, Table 1; Figure 4). T-test comparisons were completed on the mean fMRI signal from each volume of activation that passed the significance threshold (p< 0.05 corrected) for the main effect of stimulus type. All regions showed a diminished UCR on CS+UCS trials compared to the UCS alone. Most of these areas also showed a reduction in UCR amplitude on CS+UCS trials compared to CS−UCS trials. The left IPL and right anterior insula were the only regions that did not show a diminished UCR on CS+UCS compared to CS−UCS trials. In addition, the left dorsolateral PFC and left IPL 28 showed reduced UCR amplitude on CS−UCS trials compared to presentations of the UCS alone (Table 1). Percent signal change data from each of the clusters of activation that met our significance criteria were correlated with trait anxiety, UCS expectancy, and unconditioned SCR amplitude measures (Table 1; p< 0.05 Bonferroni corrected). The mean percent signal change within these brain regions did not vary with trait anxiety or unconditioned SCR. However, there was a significant correlation observed between UCS expectancy ratings and activity within the bilateral dorsolateral PFC, dorsomedial PFC, bilateral IPL, bilateral insula, and PCC (Table 1). A voxel-wise multiple linear regression analysis that accounted for stimulus type was also conducted to determine areas in which brain activity varied with trait anxiety, UCS expectancy, and SCR production. This analysis was restricted to regions that showed UCR diminution from the ANOVA with one exception. The amygdala was included, based on prior work demonstrating its role in learning-related SCR production (Cheng et al., 2003, 2006, 2007; Knight et al., 2005) even though it did not meet the significance criteria for the ANOVA. This analysis demonstrated that inter-subject trait anxiety levels explained unique variability in the activation observed within the dorsolateral PFC, dorsomedial PFC, PCC, and IPL (Table 3; Figure 5). There were no brain regions that showed a relationship with UCS expectancy that met our significance criteria. Unconditioned SCR amplitude varied with UCS−related activity within the left (r = 0.45) and right (r = 0.42) amygdala (Talairach coordinates and volume: left; -25, -4, -15 and 511mm3, right; 25, -4, -16 and 460mm3; Figure 6 a & b). Because our initial ANOVA did not demonstrate UCR diminution within the amygdala that met our 29 significance criteria, we created functional ROI from the bilateral amygdala volumes that were associated with unconditioned SCR production. These functional ROI were then used to assess UCR diminution within the amygdala. UCR diminution was observed within the bilateral amygdala (Figure 6 c & d). The amplitude of the unconditioned fMRI signal response within the amygdala was diminished on CS+UCS trials compared to UCS alone trials (left; t[20] = -2.175; right; t[20] = -1.860, p< 0.05). The UCR on CS−UCS trials fell at an intermediate level and did not differ from the UCR to CS+UCS or UCS alone trials (Figure 6 c & d). We also completed a group level regression analysis to determine whether anticipatory PFC activity varied with threat-related activity obtained from the amygdala and functional ROI from our ANOVA (regions depicted in Figures 3, 4, & 6 and Table 1). Anticipatory activity (i.e. the CR) within the dorsolateral PFC and dorsomedial PFC showed a negative relationship with threat-related (i.e. the UCR) activity on CS+UCS, but not CS−UCS trials within many of our functional ROI. This effect was observed between anticipatory right dorsolateral PFC activity and threat-related activity within the left dorsolateral PFC (Figure 7b, d) and right IPL (Figure 7a). A similar pattern was observed between anticipatory activity within 2 separate areas of the left dorsolateral PFC and the threat response within an adjacent area of the left dorsolateral PFC (Figure 7c and d). Finally, the same basic pattern was observed between anticipatory dorsomedial PFC activity and threat-related responses within the left dorsolateral PFC (Figure 7g and h), ventromedial PFC (Table 4), and bilateral amygdala (Figure 7e and f). Threat-related activity within the remaining functional ROI, including the right dorsolateral PFC, 30 dorsomedial PFC, left IPL, bilateral insula, and PCC did not vary with anticipatory activity in the PFC. Discussion The ability to identify and quickly respond to a threat is critical to survival. Associative learning processes allow one to predict impending threats to more effectively avoid or escape danger (Franchina, 1969; Kamin, 1954). Further, these processes can minimize the reaction to the threat itself (Dunsmoor et al., 2008; Knight et al., 2010; Marcos & Redondo, 1999). For example, conditioned hypoalgesia (decreased sensitivity to painful stimuli) develops during fear conditioning to reduce the pain produced by noxious stimuli (Helmstetter, 1992; Helmstetter & Bellgowan, 1993). A similar process appears to diminish the emotional response elicited by aversive stimuli during fear conditioning (Dunsmoor et al., 2008; Knight et al., 2010; Marcos & Redondo, 1999; Sarinopolous et al., 2010). The present study investigated trait anxiety, UCS expectancy, unconditioned SCR, and fMRI signal responses during Pavlovian conditioned UCR diminution to better understand the processes that influence learning-related changes in the emotional response to a threat. In the current study, we observed conditioned diminution of the unconditioned SCR. The magnitude of the unconditioned SCR was diminished on CS+UCS trials compared to the UCR produced during CS−UCS and UCS alone trials (see Figure 2b). These data demonstrate that the emotional response to an aversive stimulus is reduced when it is predictable. These findings are consistent with prior behavioral research that has demonstrated a reduction in UCR magnitude when the UCS follows a CS+ compared 31 to when the UCS follows a CS− or is presented alone (Baxter, 1966; Kimmel, 1967; Knight et al., 2011; Lykken et al., 1972; Marcos & Redondo, 1999; Redondo & Marcos, 2002). Taken together, these findings support the view that UCR diminution during Pavlovian conditioning is in part mediated by an associative learning process. Learning-related changes in UCS expectancy were also observed in the present study. UCS expectancy ratings on CS+UCS trials were consistently high across Early and Late test trials, indicating that participants expected the UCS to follow the CS+. UCS expectancy ratings to the UCS alone were relatively low on both Early and Late test trials. In contrast, UCS expectancy ratings on CS−UCS test trials increased over the course of the study such that expectancy ratings on Late test trials were larger to the CS−UCS than UCS alone (Figure 2a). These findings demonstrate that participants learned that there was a change in the relationship between the CS− and UCS over the course of the study. The increase in UCS expectancy ratings on CS−UCS trials indicates participants learned that the CS− no longer predicted the absence of the UCS, but instead that the UCS would follow the CS−. Previous research indicates that UCS expectancy modulates unconditioned autonomic responses (Dunsmoor et al., 2008; Knight et al., 2010, 2011). These studies have demonstrated that as UCS expectancy increases, unconditioned SCRs decrease (Dunsmoor et al., 2008; Knight et al., 2010, 2011). This prior work is generally consistent with investigations of the neural mechanisms of error detection. For example, brain activity decreases as the relationship between a CS and UCS becomes predictable (Fletcher et al., 2001). However, activity increases when an outcome violates expectations (Casey et al., 2000; Fletcher et al., 2001). Therefore, conscious expectations that a threat is imminent may play an important role in UCR 32 diminution. Interestingly however, the unconditioned SCR produced in response to CS−UCS trials did not diminish over the course of the study (Figure 2b). Further, unconditioned SCR amplitude did not differ between the CS−UCS and UCS alone on Late test trials, as would be predicted if UCR diminution was solely mediated by changes in UCS expectancy. Instead, the magnitude of the unconditioned SCR on CS−UCS trials remained elevated. This finding suggests that UCR diminution is not solely mediated by UCS expectancy. Instead, associative learning processes independent of UCS expectancy also appear to influence UCR diminution. This conclusion is consistent with prior work demonstrating that both UCS expectancy and an expectancy independent conditioning process influence UCR diminution (Knight et al., 2011). For example, unconditioned SCR diminution is greater to CS+UCS than CS−UCS presentations even after participants have been explicitly informed that the UCS would follow both CS+ and CS− presentations on test trials (Marcos & Redondo, 1999). Further, greater UCR diminution has been observed to CS+UCS than CS−UCS trials with equivalent UCS expectancy ratings (Knight et al., 2011). Thus, while there is strong evidence from prior work that demonstrates expectations of the UCS modulate UCR expression, learning-related processes that are independent of expectancy also provide discriminative control over the UCR. In general, these findings are consistent with prior work that has demonstrated a dissociation between UCS expectancy and SCR expression (Balderston & Helmstetter, 2010; Knight et al., 2003, 2006, 2009; Schultz & Helmstetter, 2010). In the present study, UCR diminution was observed in both SCR and fMRI data. Unconditioned SCR magnitude was diminished on CS+UCS compared to UCS alone trials, and this response pattern was paralleled by the fMRI signal response within the 33 dorsolateral PFC, dorsomedial PFC, anterior insula, IPL, PCC, and amygdala. The unconditioned SCR and fMRI signal within most of these brain regions also showed diminution on CS+UCS compared to CS−UCS trials (Table 1; Figures 2 & 3). Diminution of the unconditioned fMRI signal response within each of these brain regions has been observed in previous neuroimaging studies (Dunsmoor et al., 2008; Knight et al., 2010). For example, the UCR diminution observed within the dorsomedial PFC in the present study overlaps with the anterior cingulate (Dunsmoor et al., 2008) and dorsomedial PFC (Knight et al., 2010) activations observed in prior work. Further, the UCR diminution observed within dorsolateral PFC (Dunsmoor et al., 2008; Knight et al., 2010), IPL (Dunsmoor et al., 2008; Knight et al., 2010), PCC (Knight et al., 2010), insula (Knight et al., 2010), and amygdala (Dunsmoor et al., 2008) in prior studies largely overlaps with the activity observed in the present study. The conditioning procedures employed by these prior studies differ somewhat from those used in the present study. For example, Knight et al. (2010) demonstrated diminution of the unconditioned SCR and fMRI signal response by varying the volume of auditory CS presentations above and below the perceptual detection threshold. The UCR was diminished when the UCS followed a perceived versus unperceived CS+ (Knight et al., 2010). Dunsmoor et al. (2008) investigated differences in unconditioned SCR and fMRI signal amplitude using continuous reinforcement (i.e. 100% pairing of the CS and UCS) and partial reinforcement (i.e. 50% pairing of the CS and UCS) conditioning procedures, and demonstrated greater UCR diminution during continuous than partial reinforcement (Dunsmoor et al., 2008). 34 The methodology of the present research is similar to that used in the Dunsmoor et al. (2008) study in that the CS− was partially paired with the UCS during acquisition. More specifically, the CS− was paired with the UCS on one (i.e. the CS−UCS test trial) out of every 9 trials (approximately 11% CS and UCS pairing rate) during acquisition blocks. The CS− was then paired with the UCS on every trial during the test block (100% pairing rate). Given the nature of this type of study, the CS− must be paired with the UCS to assess UCR diminution. However, by pairing the CS− and UCS the contingencies between the two stimuli change, and participants learn that the CS− is paired with the UCS on a percentage of the trials. Such learning is apparent in the UCS expectancy ratings depicted in Figure 2a. The intermittent pairing of the CS− and UCS during acquisition was intended to increase the number of CS−UCS trials in which low UCS expectancy was reported because prior work indicates that UCS expectancies modulate UCR amplitude (Knight et al., 2010, 2011). This effect is also illustrated in the current study. A significant negative correlation was observed between UCS expectancy and activity within several brain regions that showed UCR diminution (Table 1). However, this relationship was not apparent in our group level regression analysis. Together, these findings indicate UCS expectancy varies with the fMRI signal within these ROI, but does not explain unique variance in the fMRI data when other regressors (i.e. stimulus type) are included in the model. In combination with prior research (Dunsmoor et al., 2008; Knight et al., 2010), the current findings support the view that differences in the emotional response (indexed with SCR) to predictable versus unpredictable threats are mediated by associative learning processes that are supported by a network of brain regions that includes the amygdala and PFC (Figures 3, 4, & 6). 35 Prior work suggests that prefrontal processes support contingency learning (Knight et al., 2004; Phillips et al., 2003), modulation of the emotional response (Delgado et al., 2008; Milad et al., 2004; Ochsner et al., 2002), and the integration of emotional information (Phillips et al., 2003). In line with work that suggests an interconnected dorsal and ventral system processes and responds to emotional stimuli (Phillips et al., 2003), we found evidence that suggests these dorsal and ventral systems mediate the UCR diminution observed during Pavlovian conditioning. The dorsal system appears to integrate cognitive processes that can be affected by emotional input (Phillips et al., 2003), and the present study demonstrated UCR diminution in multiple dorsal system brain regions (i.e. dorsolateral PFC, dorsomedial PFC, and IPL; Figure 3). Thus, the emotion-related processes supported by this dorsal system may mediate UCR diminution. We also observed UCR diminution within the amygdala as well as a strong relationship between amygdala activity and the unconditioned SCRs that showed conditioned UCR diminution (Figure 6). This finding is consistent with the view that a ventral neural system assesses the emotional significance of environmental stimuli and controls expression of the emotional response (Phillips et al., 2003). The amplitude of the fMRI signal response within many of the brain regions showing UCR diminution fluctuated with inter-subject variations in trait anxiety. The voxel-wise multiple linear regression analysis demonstrated that as trait anxiety increased, UCR activity increased within the dorsolateral PFC, dorsomedial PFC, PCC, and IPL (Figure 5 & Table 3). Although the multiple regression analysis demonstrated a relationship between trait anxiety and the magnitude of ROI activity, a similar effect was not observed in the correlation analysis (Table 1). The regression analysis indicates that 36 activity within a subset of the voxels within these ROI vary with trait anxiety. This effect was not observed in the correlation analysis, because the voxels that showed a relationship between trait anxiety and ROI activity were averaged together with voxels that did not show the same relationship, diluting the effect. In general, the multiple regression findings are generally consistent with prior work suggesting that trait anxiety modulates activity within these brain regions as threatening stimuli are assessed (Klumpp et al., 2011). Related work suggests that trait anxiety levels influence the activity within brain regions that support regulatory control processes (Basten et al., 2011; Sehlmeyer et al., 2011). For example, dorsomedial PFC activity shows a negative relationship with trait anxiety during the extinction of conditioned fear (Sehlmeyer et al., 2011). Other research has shown that functional connectivity between the dorsomedial PFC and the amygdala is weaker in high compared to low anxious individuals (Kim et al., 2011). The present findings are consistent with prior work that suggests trait anxiety modulates activity within dorsal brain regions that support emotion-related processes. Previous research has also shown that changes in the magnitude of the unconditioned fMRI signal response within the dorsolateral PFC, dorsomedial PFC, and insula are paralleled by changes in autonomic activity (Knight et al., 2010). In the present study, we found the amplitude of UCR activity within the amygdala varied with the amplitude of the unconditioned SCR. Our data suggest that the amygdala also plays an important role in the learning-related modulation of the unconditioned SCR. These findings are consistent with prior research that demonstrated the amygdala and PFC influence the expression of the emotional response (Cheng et al., 2003, 2006; Delgado et al., 2008; Knight et al., 2005; Milad et al., 2004, 2007; Sarinopoulos et al., 2010). 37 Prior work suggests that the PFC regulates activity within brain regions (e.g. amygdala & insula) that support emotional expression (Delgado et al., 2008; Milad et al., 2004, 2007; Sarinopoulos et al., 2010). For example, anticipatory PFC activity is negatively correlated with amygdala and insula responses to aversive stimuli (Sarinoplous et al., 2010). The findings from the present study are relatively consistent with this prior work. In the present study, an inverse relationship was observed between anticipatory PFC and threat-related activity within several brain regions showing UCR diminution. As anticipatory dorsomedial and dorsolateral PFC activity increased, threatrelated activity within the dorsolateral PFC, ventromedial PFC, IPL, and amygdala decreased on CS+UCS trials (Table 4; Figure 7). These findings are generally consistent with the view that the PFC supports emotion-related processes, and that anticipatory activity affects threat-related responses within the PFC-amygdala circuit. In summary, learning-related changes in the unconditioned SCR and fMRI signal response were observed during Pavlovian fear conditioning. The current findings replicate prior work demonstrating UCR diminution using functional neuroimaging in conjunction with behavioral measures (e.g. SCR and UCS expectancy ratings) during conditioning (Dunsmoor et al., 2008; Knight et al., 2010). Investigating the neurobiological processes that mediate conditioned UCR diminution may provide a starting point to better understand the emotional dysregulation that characterizes many anxiety disorders (Grillon, 2002; Davis et al., 2009; Kim & Jung, 2006; Milad et al., 2006). For example, prior investigations of the neurobiological markers of anxiety suggest that insufficient top-down inhibitory control (Klumpp et al., 2011; Nitschke et al., 2006; Rauch et al., 2006; Schienle et al., 2010) may result in hypersensitivity of the 38 subcortical brain areas (e.g. the amygdala) that control the peripheral expression of emotion (Etkin & Wager, 2007; Milad et al., 2006, 2009; Rauch et al., 2006). Several brain regions that integrate cognitive processes and modulate emotional responses exhibited UCR diminution (i.e. dorsolateral PFC and dorsomedial PFC). UCR diminution was also observed within the PCC, anterior insula, and IPL. Furthermore, many of the brain regions that demonstrated UCR diminution in this study have also been implicated in regulating autonomic responses associated with affective states and emotional behavior. The findings from the present study are consistent with the view that anticipatory activity within the PFC may inhibit threat-related activity within this neural circuit. In turn, the learning-related changes in UCR amplitude within these brain regions may interact with the amygdala to modulate unconditioned SCR production. The present findings provide a better understanding of the psychological processes and neural circuitry that mediate the threat-related emotional response. Acknowledgements: This research was supported by the University of Alabama at Birmingham Faculty Development Grant Program. 39 References Balderston, N. L., & Helmstetter, F. J. (2010). Conditioning with masked stimuli affects the timecourse of skin conductance responses. Behavioral Neuroscience, 124(4), 478–489. Basten, U., Stelzel, C., Fiebach, C.J. (2011). Trait anxiety modulates the neural efficiency of inhibitory control. Journal of Cognitive Neuroscience, 10, 3132-3145. Baxter, R. (1966). Diminution and recovery of the UCR in delayed and trace classical GSR conditioning. Journal of Experimental Psychology, 71(3), 447-451. Büchel, C., Morris, J., Dolan, R.J., & Friston, K.J. (1998). Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron, 20, 947-957. Casey, B. J., Thomas, K.M., Welsch, T.F., Badgaiyan, R.D., Eccard, C.H., Jennings, J.R., & Crone, E.A. (2000). Dissociation of response conflict, attentional selection and expectancy with functional magnetic resonance imaging. Proceedings of the National Academy of Sciences, 97(15), 8728–8733. Cheng, D. T., Knight, D. C., Smith, C. N., Stein, E. A., & Helmstetter, F. J. (2003). Functional MRI of human amygdala activity during Pavlovian fear conditioning: Stimulus processing versus response expression. Behavioral Neuroscience, 117 (1), 3-10. Cheng, D. T., Knight, D. C., Smith, C. N., Stein, E. A., & Helmstetter, F. J. (2006). Human amygdala activity during the expression of fear responses. Behavioral Neuroscience, 120 (6), 1187-1195. Cheng, D. T., Richards, J., & Helmstetter, F. J. (2007). Activity in the human amygdala corresponds to early, rather than late period autonomic responses to a signal for shock. Learning & Memory, 14 (7), 485-490. Cox, R.W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162-173. Critchley, H.D., Mathia, C.J., & Dolan, R.J. (2001). Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron, 29, 537-545. Davis, M., Walker, D.L., Miles, L., & Grillon, C. (2009). Phasic vs. sustained fear in rats and humans: role of the extended amygdala in fear vs. anxiety. Neuropsychopharmacology Reviews, 1-31. Davis, M., & Whalen, P.J. (2001). The amygdala: vigilance and emotion. Molecular Psychiatry, 6, 13-34. 40 Delgado, M.R., Nearing, K.I., LeDoux, J.E., & Phelps, E.A. (2008). Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron, 59, 829-838. Domjan, M. (2005). Pavlovian conditioning: A functional perspective. Annual Review of Psychology, 56, 179-206. Dunsmoor, J.E., Bandettini, P.A., & Knight, D.C. (2007). Impact of continuous versus intermittent CS−UCS pairing on human brain activation during Pavlovian fear conditioning. Behavioral Neuroscience, 121, 635-642. Dunsmoor, J.E., Bandettini, P.A., & Knight, D.C. (2008). Neural correlates of unconditioned response diminution during Pavlovian conditioning. NeuroImage, 40, 811-817. Etkin, A. & Wager, T.D. (2007). Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry, 164, 1476-1488. Fletcher, P. C., Anderson, J. M., Shanks, D. R., Honey, R., Carpenter, T. A., Donavan, T., Papadakis, N., & Bullmore, E.T. (2001). Responses of human frontal cortex to surprising events are predicted by formal associative learning theory. Nature Neuroscience, 4(10), 1043-1048. Forman, S.D., Cohen, J.D., Fitzgerald, M., Eddy, W.F., Mintun, M.A., & Noll, D.C. (1995). Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster size threshold. Magnetic Resonance in Medicine, 33, 636–647. Franchina, J. J. (1969). Escape behavior and shock intensity: Within subject versus between-groups comparisons. Journal of Comparative and Physiological Psychology, 69(2), 241–245. Grillon, C. (2002). Startle reactivity and anxiety disorders: Aversive conditioning, context, and neurobiology. Biological Psychiatry, 52, 958-975. Grös, D.F., Antony, M.M., Simms, L.J., & McCabe., R.E. (2007). Psychometric Properties of the State–Trait Inventory for Cognitive and Somatic Anxiety (STICSA): Comparison to the State–Trait Anxiety Inventory (STAI). Psychological Assessment 19(4), 369-381. Hartley, C.A., & Phelps, E.A. (2010). Changing fear: The neurocircuitry of emotion regulation. Neuropsychopharmacology, 35, 136-146. Helmstetter, F.J. (1992). The amygdala is essential for the expression of conditional hypoalgesia. Behavioral Neuroscience, 106(3), 518-528. 41 Helmstetter, F. J., & Bellgowan, P. S. (1993). Lesions of the amygdala block conditional hypoalgesia on the tail flick test. Brain Research, 612(1–2), 253–257. Herwig, U., Baumgartner, T., Kaffenberger, T., Bruhl, A., Kottlow, M., Schreiter-Gasser, Abler, B., Jäncke L., & Rufer M. (2007). Modulation of anticipatory emotion and perception processing by cognitive control. NeuroImage, 37, 652-662. Indovina, I., Robbins, T.W., Núñez-Elizalde, A.O., Dunn, B.D., & Bishop, S.J. (2011). Fear-conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron, 69, 563-571. Kahnt, T., Heinzle, J., Park, S.Q., & Haynes, J.D. (2011). Decoding different roles for vmPFC and dlPFC in multi-attribute decision making. NeuroImage, 56, 709-715. Kamin, L. J. (1954). Traumatic avoidance learning: The effects of CS−US interval with a trace-conditioning procedure. Journal of Comparative and Physiological Psychology, 47(1), 65–72. Kim, M.J., Gee, D.G., Loucks, R.A., Davis, F.C., & Whalen, P.J. (2011). Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cerebral Cortex, 7, 1667-1673. Kim., J.J., & Jung, M.W. (2006). Neural circuits and mechanisms involved in Pavlovian fear conditioning: A critical review. Neuroscience and Biobehavioral Reviews, 30, 188-202. Kimmel, E. (1967). Judgments of UCS intensity and diminution of the UCR in classical GSR conditioning. Journal of Experimental Psychology, 73, 532-543. Klumpp, H., Ho, S.S., Taylor, S.F., Phan, K.L., Abelson, J.L., & Liberzon, I. (2011). Trait anxiety modulates anterior cingulate activation to threat interference. Depression and Anxiety, 28, 194-201. Knight, D. C., Smith, C. N., Stein, E. A., & Helmstetter, F. J. (1999). Functional MRI of human Pavlovian fear conditioning: Patterns of activation as a function of learning. NeuroReport, 10 (17), 3665-3670. Knight, D.C., Nguyen, H.T., & Bandettini, P.A. (2003). Expression of conditional fear with and without awareness. Proceedings of the National Academy of Sciences, 100(25), 15280-15283. Knight, D.C., Cheng, D.T., Smith, C.N., Stein, E.A., & Helmstetter, F.J. (2004). Neural substrates mediating human delay and trace fear conditioning. Journal of Neuroscience, 24, 218-228. Knight, D. C., Nguyen, H. T., & Bandettini, P. A. (2005). The role of the human amygdala in the production of conditioned fear responses. NeuroImage, 26, 11931200. 42 Knight, D.C., Nguyen, H.T., & Bandettini, P.A. (2006). The role of awareness in delay and trace fear conditioning in humans. Cognitive, Affective, and Behavioral Neuroscience, 6(2), 157-162. Knight, D.C., Waters, N.S., & Bandettini, P.A. (2009). Neural substrates of explicit and implicit fear memory. NeuroImage, 45, 208-214. Knight, D.C. Waters, N.S., King, M.K., & Bandettini, P.A. (2010). Learning-related diminution of unconditioned SCR and fMRI signal responses. NeuroImage, 49, 843-848. Knight, D. C., Lewis, E. P., & Wood, K. H. (2011). Conditioned diminution of the unconditioned SCR. Behavioral Neuroscience, 124 (4), 626-631. Knight, D. C. & Wood, K. H. (2011). Investigating the neural mechanisms of aware and unaware fear memory with fMRI. Journal of Visualized Experiments (56), e3083, DOI:10.39791/2083. LaBar, K.S., Gatenby, J.C., Gore, J.C., LeDoux, J.E., & Phelps, E.A. (1998). Human amygdala activation during conditioned fear acquisition and extinction: a mixedtrial fMRI study. Neuron, 20, 937-945. Linnman, C., Rougemont-Bücking, A., Beucke, J. C., Zeffiro, T. A., & Milad, M. R. (2011). Unconditioned responses and functional fear networks in human classical conditioning. Behavioral Brain Research, 221 (1), 237-245. Loftus, G.R., & Masson, M.E.J. (1994). Using confidence intervals in within-subject designs. Psychonomic Bulletin & Review, 4, 476-490. Lykken, D.T., Macindoe, Y., & Tellegen, A. (1972). Perception: Autonomic responses to shock as a function of predictability in time and locus. Psychophysiology, 9, 318333. Lykken, D.T., & Tellegan, A. (1972). On the validity of the perception hypothesis. Psychophysiology, 11, 125.132. Marcos, J.L., & Redondo, J. (1999). Effects of conditioned stimulus presentation on diminution of the unconditioned response in aversive classical conditioning. Biological Psychology, 50, 89-102. Milad, M.R., Vidal-Gonzalez, I., & Quirk, G.J. (2004). Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behavioral Neuroscience, 118, 389-394. Milad, M.R., Rauch, S.L., Pitman, R.K., & Quirk, G.J. (2006). Fear extinction in rats: Implications for human brain imaging and anxiety disorders. Biological Psychology, 73, 61-71. 43 Milad, M.R., Quirk, G.J., Pitman, R.K., Orr, S.P., Fischl, B., & Rauch, S.L. (2007). A role for the human dorsal anterior cingulate cortex in fear expression. Biological Psychology, 62, 1191-1194. Milad, M.R., Pitman, R.K., Ellis, C.B., Gold, A.L., Shin, L.M., Lasko, N.B., Zeidan, M.A., Handwerger, K., Orr, S.P., & Rauch, S.L. (2009). Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry, 66, 1075-1082. Morgane, P.J., Galler, J.R., & Mokler, D.J. (2005). A review of systems and networks of the limbic forebrain/limbic midbrain. Progress in Neurobiololgy, 75, 143–160. Nitschke, J.B., Dixon, G.E., Sarinopoulos, I., Short, S.J., Cohen, J.D., Smith, E.E., Kosslyn, S.M., Rose, R.M., & Davidson, R.J. (2006). Altering expectancy dampens neural response to aversive taste in primary taste cortex. Nature Neuroscience, 9, 435-442. Ochsner, K.N., Bunge, S.A., Gross, J.J., & Gabrieli, J.D. (2002). Rethinking feelings: an fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience, 14, 1215-1229. Peeke, S.C., & Grings, W.W. (1968). Magnitude of UCR as a function of variability in the CS−UCS relationship. Journal of Experimental Psychology, 77, 1-6. Phillips, M.L., Drevets, W.C., Rauch, S.L., & Lane, R. (2003). Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry, 54, 504-514. Rauch, S.L., Shin, L.M., & Phelps, E.A. (2006). Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research—past, present, and future. Biological Psychiatry, 60, 376–382. Redondo, J., & Marcos, J.L. (2002). Effects of CS−US interval on unconditioned response diminution in human heart rate classical conditioning. Journal of Psychophysiology, 17, 30-38. Rescorla, R. A. (1988). Behavioral studies of Pavlovian conditioning. Annual Review of Neuroscience, 11, 329-352. Rescorla, R. A., & Wagner, A. R. (1972). A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In A. H. Black & W. F. Prokasy (Eds.), Classical conditioning II: current research and theory (pp. 6499). New York: Appleton-Century-Crofts. Rust, J. (1976). Unconditioned response diminution in the skin resistance response. The Journal of General Psychology, 95, 77-84. 44 Saad, Z.S., Chen, G., Reynolds, R.C., Christidis, P.P., Hammett, K.R., Bellgowan, P.S.F., & Cox, R.W. (2006). Functional imaging analysis contest (FIAC) analysis according to AFNI and SUMA. Human Brain Mapping, 27, 417-424. Sarinopoulos, I., Grupe, D.W., Mackiewicz, K.L., Herrington, J.D., Lor, M., Steege, E.E., & Nitschke, J.B. (2010). Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cerebral Cortex, 20, 929940. Schienle, A., K chel, A., Ebner, F., Reishofer, G., & Schäfer, A. (2010). Neural correlates of intolerance of uncertainty. Neuroscience Letters, 479 (3), 272-276. Schultz, D.H. & Helmstetter, F.J. (2010). Classical conditioning of autonomic fear responses is independent of contingency awareness. Journal of Experimental Psychology, 36(4), 495-500. Sehlmeyer, C., Dannlowski, U., Schöning, S., Kugel, H., Pyka, M., Pfleiderer, B., Zwitserlood, P., Schiffbauer, H., Heindel, W., Arolt, V., & Konrad, C. (2011). Neural correlates of trait anxiety in fear extinction. Psychological Medicine, 41, 789-798. Spielberger, C.D. (1983). State-Trait Anxiety Inventory for Adults. Redwood City, CA: Mind Garder. Talairach, J., & Tournoux, P. (1988). Co-planar Stereotaxic Atlas of the Human Brain. Thieme, New York. Viinikainen, M., Jääskeläinen, I.P., Alexandrov, Y., Balk, M.H., Autti, T., & Sams, M. (2010). Nonlinear relationship between emotional valence and brain activity: Evidence of separate negative and positive valence dimensions. Human Brain Mapping, 31, 1030-1040. Volz, K.G., Schubotz, R.I., & von Cramon, D.Y. (2003). Predicting events of varying probability: uncertainty investigated by fMRI. NeuroImage, 19, 271-280. Wagner, A. R. & Brandon, S. E. (1989). Evolution of a structured connectionist model of Pavlovian conditioning (AESOP). In S. B. Klein & R. R. Mowrer (Eds.), Contemporary Learning Theories: Pavlovian Conditioning and the Status of Traditional Learning Theory. (pp. 149-189). Hillsdale, NJ: Erlbaum. 45 Table 1. Regions showing conditioned diminution of the UCR. Talairach coordinates Region Vol (mm3) Main Effect of Stimulus type Dorsolateral PFC x y CS+UCS vs. CS−UCS CS−UCS vs. UCS alone CS+UCS vs. UCS alone Trait SCR UCS Expectancy z t t t r r r Right 3,668 30.3 44.0 27.4 − 6.03 n.s. − 6.37 0.16 − 0.01 − 0.28 Right 14,229 40.3 12.8 36.6 − 4.23 n.s. − 5.18 0.19 − 0.01 − 0.42* Left 11,710 − 37.7 10.2 38.1 − 3.26 − 2.96 − 4.67 0.12 0.06 − 0.48* Left 1,110 − 32.6 50.1 19.2 − 3.20 n.s. − 4.75 0.19 0.01 − 0.38* Left 908 − 21.1 41.0 40.2 − 4.53 n.s. − 2.89 0.27 0.00 − 0.28 22,771 1.0 20.3 38.6 − 3.84 n.s. − 5.07 0.23 0.12 − 0.47* 3,210 42.2 − 52.5 42.6 − 3.65 n.s. − 4.77 0.33 0.06 − 0.38* Dorsomedial PFC Inf. Parietal Lobule Right Right 892 48.5 − 42.4 25.0 − 3.50 n.s. − 3.68 0.15 0.04 − 0.27 Left 4,672 − 42.2 − 51.1 42.9 n.s. − 4.69 − 5.29 0.22 0.08 − 0.54* Right 1,398 38.4 15.3 0.3 n.s. n.s. − 3.70 0.24 0.08 − 0.41* Left 4,731 − 37.5 13.7 − 0.8 − 3.54 n.s. − 4.67 0.28 0.26 − 0.49* 11,381 − 1.7 − 34.4 32.5 − 4.08 n.s. − 5.05 0.21 0.06 − 0.39* 12.0 48.9 13.6 − 4.58 n.s. n.s. 0.05 0.13 − 0.26 Anterior Insula Posterior Cingulate Stimulus x Trial Interaction Ventromedial PFC 656 Early test Trials Late test Trials n.s. n.s. n.s. 0.14 0.10 0.09 Note. Location, volumes, and coordinates from Talairach and Tournoux (1988) for the center of mass for areas of activation. Significance criteria: ANOVA F[21] > 5.63, p < 0.05 (corrected); t[20] p < 0.05 (corrected). Significance criteria for two-tailed correlations: * indicates p < 0.05 (corrected). 46 Table 2. Regions showing change over time. Region 3 Talairach coordinates x z y Hemisphere Vol (mm ) Right 2,379 22.0 42.2 29.3 Right 1,308 22.7 18.4 47.0 Left 9,230 -40.4 11.6 33.2 Dorsomedial PFC Left 22,211 -2.2 31.2 27.2 Posterior Cingulate Left 5,628 -1.3 -47.6 26.2 Anterior Insula Right 1,991 38.7 18.6 0.1 Dorsolateral PFC Left 2,068 -35.9 13.4 -1.7 Note. Location, volumes, and coordinates from Talairach and Tournoux (1988) for the center of mass for areas of activation. Significance criteria: F[21] > 9.01; p < 0.05 (corrected). 47 Table 3. Regional activity varying with trait anxiety. Talairach coordinates Region 3 Hemisphere Vol (mm ) Right 702 36.2 9.4 46.8 0.33 Right 429 36.7 28.1 36.8 0.38 Dorsomedial PFC Left 1,155 − 2.4 14.4 50.3 0.34 Posterior Cingulate Left 711 − 1.1 − 43.5 24.6 0.35 Inf. Parietal Lobule Right 992 42.2 − 53.6 43.3 0.39 Dorsolateral PFC x y z Trait r Left 744 − 40.1 − 59.4 42.6 0.33 Note. Location, volumes, and coordinates from Talairach and Tournoux (1988) for the center of mass for areas of activation. Significance criteria: p < 0.05 (corrected). 48 Table 4. Regions showing a relationship between anticipatory and threat-related activity. UCR DIMINUTION FUNCTIONAL ROIs ANTICIPATORY BRAIN ACTIVATION Talairach coordinates Region Vol (mm3) x y Talairach coordinates z Region Main Effect of Stimulus type Dorsolateral PFC Vol (mm3) x y z 749 29.1 21.8 44.7 Dorsomedial PFC2g 8,864 7.9 − 9.2 41.3 Dorsomedial PFC2dh 4,177 7.9 12.3 41.4 Dorsolateral PFC Left 11,710 − 37.7 10.2 38.1 Left 1,110 − 32.6 50.1 19.2 Left 908 − 21.1 41.0 40.2 ↔ ↔ ↔ Right1b Dorsolateral PFC Right1d Inf. Parietal Lobule Right 2,114 30.1 14.4 42.1 Left d 1,219 − 35.1 20.9 34.9 Left c 1,035 − 33.0 46.3 20.3 638 27.0 20.3 41.0 1,002 7.4 20.9 56.6 Dorsomedial PFC2f 645 16.2 − 5.7 45.9 Dorsomedial PFC2e 12,121 6.7 − 13.1 45.4 Dorsolateral PFC 3,210 42.2 − 52.5 42.6 ↔ Right1a Stimulus x Trial Interaction ↔ Ventromedial PFC 656 12.0 48.9 13.6 Dorsomedial PFC Regression of Unconditioned SCR Amygdala Right 460 24.9 − 4.3 − 16.1 Left 511 − 24.9 − 4.4 − 14.8 ↔ ↔ Note. Location, volumes, and coordinates from Talairach and Tournoux (1988) for the center of mass for areas of activation. Significance criteria: t[20] > 2.85, p < 0.05 (corrected). A stimulus (CS+UCS vs CS−UCS) x UCR amplitude (from ROI on left side of table) interaction was observed in the anticipatory response (i.e. the CR) within the dorsolateral and dorsomedial PFC (right side of the table). Numbers (1 & 2) denote areas of overlap within the regions showing an interaction effect. Letters (a-h) correspond to images presented in Figure 7. 49 Acquisition Trials Test Trials CS+ CS+ UCS UCS CS- CS- UCS 0 10 20 30 40 Time (Seconds) UCS 50 No CS UCS 0 10 20 30 40 Time (Seconds) 50 Figure 1. Conditioning procedure. Acquisition blocks consisted of CS+ (8 trials), CS− (8 trials), and test trials (1 CS+UCS, 1 CS−UCS, and 1 UCS alone trial). Four acquisition blocks were presented, followed by a block of test trials. The test trial block consisted of 10 CS+UCS trials, 10 CS−UCS trials, and 10 UCS alone trials. Stimuli were counterbalanced and presented in a pseudorandom order such that no more than two trials of the same stimulus were consecutively presented. 50 UCS Expectancy 100 a 80 60 40 CS+UCS CS-UCS UCS alone 20 SCR 0.20 b 0.15 0.10 0.05 1-7 8-14 Trials Figure 2. UCS Expectancy and Unconditioned SCR. a) Learning-related differences in UCS expectancy. UCS expectancy on Early test trials (1-7) was higher to the CS+UCS than to CS−UCS and UCS alone trials. UCS expectancy on Late test trials (8-14) remained high for the CS+UCS, while UCS expectancy during the CS−UCS increased such that ratings were greater than those for the UCS alone. b) Learning-related changes in unconditioned SCR expression were also observed. Unconditioned SCRs were diminished on CS+UCS trials compared to CS−UCS and UCS alone trials. No differences were observed between CS−UCS and UCS alone trials. Error bars reflect SEM after adjusting for between-subject variance (Loftus & Masson, 1994). 51 Posterior Cingulate 0.5 0.4 fMRI signal (% ) fMRI signal (% ) Dorsomedial PFC * * 0.3 0.2 0.1 CS+UCS CS-UCS 0.5 0.4 0.1 CS+UCS UCS alone fMRI signal (%) fMRI signal (% ) x = -5 * CS-UCS * 0.2 0.1 0.4 0.3 * * 0.2 0.1 0.0 CS+UCS CS-UCS UCS alone CS+UCS CS-UCS 0.5 * 0.4 z = 47 * 0.3 UCS alone IPL fMRI signal (% ) fMRI signal (% ) IPL 0.2 0.1 * 0.4 * 0.3 0.2 0.1 0.0 CS+UCS CS-UCS UCS alone CS+UCS CS-UCS * 0.5 0.4 0.3 0.2 Right 0.1 CS+UCS CS-UCS UCS alone Insula UCS alone Left z = -5 fMRI signal (% ) Insula fMRI signal (% ) UCS alone Dorsolateral PFC 0.5 0.3 * 0.2 Dorsolateral PFC 0.4 * 0.3 0.5 * 0.4 * 0.3 0.2 0.1 CS+UCS CS-UCS UCS alone Figure 3. UCR diminution within the fMRI signal response. Significant diminution of the unconditioned fMRI signal response was observed within several brain regions (see Table 1) including the prefrontal cortex (PFC), inferior parietal lobule (IPL), and insula during test trials. UCR amplitude within each of these regions was reduced when the UCS followed the CS+ (i.e. CS+UCS trials) compared to when the UCS was presented alone. Many of these regions also showed diminished UCRs on CS+UCS versus CS−UCS trials. Graphs reflect the mean amplitude (% signal change) of all voxels within volumes of activation. Error bars reflect SEM after adjusting for between-subject variance (Loftus & Masson, 1994). Asterisk indicates significant difference. 52 CS+UCS CS-UCS UCS alone fMRI signal (% ) 0.4 x = 11 0.2 0.0 -0.2 1-7 8-14 Trials Figure 4. Stimulus x trial interaction within the ventromedial PFC. The unconditioned fMRI signal response within the ventromedial PFC was larger to the CS−UCS than CS+UCS and UCS alone on Early, but not Late test trials. Graph reflects the mean amplitude (% signal change) of all voxels within the volume of activation. Error bars reflect SEM after adjusting for between-subject variance (Loftus & Masson, 1994). 53 Dorsolateral PFC Dorsomedial PFC 1.0 fMRI signal (% ) fMRI signal (% ) 1.0 0.5 0.0 -0.5 0.0 -0.5 20 25 30 35 40 45 20 25 30 35 Trait Anxiety Trait Anxiety IPL IPL 40 45 40 45 1.0 0.5 Right Left z = 45 0.0 -0.5 fMRI signal (% ) 1.0 fMRI signal (% ) 0.5 0.5 0.0 -0.5 20 25 30 35 40 45 20 Trait Anxiety 25 30 35 Trait Anxiety Figure 5. Trait anxiety and the unconditioned fMRI signal response. UCR magnitude within several regions (see Table 3) including the dorsolateral PFC, dorsomedial PFC, and IPL varied with inter-subject differences in trait anxiety, such that as anxiety level increased UCR magnitude increased. 54 Right Left y = -6 fMRI signal (% ) fMRI signal (% ) a 0.4 0.2 0.0 -0.2 0.0 0.1 0.2 0.4 b 0.2 0.0 -0.2 0.3 0.0 0.15 c * 0.10 0.1 0.2 0.3 SCR fMRI signal (% ) fMRI signal (% ) SCR 0.05 0.00 -0.05 0.15 d * 0.10 0.05 0.00 -0.05 CS+UCS CS-UCS UCS alone CS+UCS CS-UCS UCS alone Figure 6. Relationship between amygdala and unconditioned SCR. a & b) A linear relationship was observed between the unconditioned SCR and the unconditioned fMRI signal response within the amygdala. These findings suggest the amygdala plays an important role in the control of unconditioned SCRs during Pavlovian conditioning. c & d) The volumes of left and right amygdala activation that correlated with unconditioned SCR were used as functional ROI to determine whether the amygdala UCR was diminished on CS+UCS trials. Learning-related changes in the unconditioned fMRI signal response were observed within the amygdala. The amplitude of the unconditioned fMRI signal response was diminished on CS+UCS trials compared to UCS alone trials. Error bars reflect SEM after adjusting for between-subject variance (Loftus & Masson, 1994). 55 Relationship between Anticipatory and Threat-related Activity dmPFC CR↔L. Amyg UCR dlPFC CR↔IPL UCR r = -.72, p<0.05; r = .27, p = n.s. a r = -.39, p = n.s.; e z = 38 x = 13 dlPFC CR↔dlPFC UCR r = -.71, p<0.05; dmPFC CR↔R. Amyg UCR r = .47, p<0.05 b r = -.58, p<0.05; x = 13 dlPFC CR↔dlPFC UCR r = -.58, p<0.05; dmPFC CR↔dlPFC UCR r = .62, p<0.05 c r = -.50, p<0.05; r = .57, p<0.05 g z = 21 x = 13 dlPFC CR↔dlPFC UCR r = -.71, p<0.05; dmPFC CR↔dlPFC UCR r = .34, p = n.s. d r = -.70, p<0.05; r = .41, p = n.s. h z = 38 x = 13 Right Left Dorsolateral PFC CS+UCS CS-UCS Dorsomedial PFC r = 0.37, p = n.s. dlPFC UCR dlPFC UCR r = .56, p<0.05 f z = 38 0.8 0.6 0.4 0.2 0.0 -0.2 -0.4 -0.6 -0.8 r = .67, p<0.05 r = -0.71, p < 0.05 -0.2 0.0 0.2 0.4 0.6 0.8 r = 0.41, p = n.s. 0.8 0.6 0.4 0.2 0.0 -0.2 -0.4 -0.6 -0.8 r = -0.70, p < 0.05 -0.2 dlPFC CR 0.0 0.2 0.4 dmPFC CR 56 0.6 0.8 Figure 7. Relationship between anticipatory and threat-related activity. Threat-related activity, extracted from the ROI depicted in Table 1 & Figures 3, 4, & 6, was included in a regression analysis to investigate differences in the relationship between anticipatory activity and threat-related activity (% signal change) on CS+UCS and CS−UCS trials. Differences (CS+UCS vs CS−UCS) in the relationship between anticipatory (i.e. CR) and threat-related (i.e. UCR) activity were observed in several areas of the PFC (a-h). These findings suggest that prefrontal anticipatory activity inhibits the threat-related response on CS+UCS trials within many of the brain regions that showed UCR diminution. Correlation values comparing anticipatory and threat-related responses on CS+UCS (blue circles) and CS−UCS (red triangles) trials are presented above the brain images. Correlation values above image (d) represent the activation observed within the left dlPFC, while correlation values for the right dorsolateral (dlPFC) and dorsomedial (dmPFC) prefrontal cortex are presented in the bottom graphs. Talairach coordinates for the depicted areas of activation are presented in Table 4 and labeled with the letters (a-h) corresponding to each image above. 57 58 NEURAL SUBSTRATES UNDERLYING LEARNING-RELATED CHANGES OF THE UNCONDITIONED FEAR RESPONSE KIMBERLY H. WOOD, DYSTANY KUYKENDALL, LAWRENCE W. VER HOEF, AND DAVID C. KNIGHT Submitted to The Open Neuroimaging Journal Format adapted for dissertation Abstract The ability to predict an impending threat during Pavlovian conditioning diminishes the emotional response that is produced once the threat is encountered. Diminution of the threat response appears to be mediated by somewhat independent associative learning and expectancy-related processes. Therefore, the present study was designed to better understand the neural mechanisms that support the associative learning processes that influence the threat-related emotional response. Healthy volunteers participated in a Pavlovian fear conditioning procedure in which trait anxiety, expectation of the unconditioned stimulus (UCS expectancy), skin conductance response (SCR), and functional magnetic resonance imaging (fMRI) signal were assessed. UCS expectancy ratings were higher on predictable trials of the UCS compared to unpredictable trials. Threat-related SCR expression was diminished on predictable trials vs. unpredictable trials of the UCS. UCR diminution was also observed within left dorsolateral PFC, dorsomedial PFC, ventromedial PFC, and left anterior insula, whereas potentiation of the threat-related fMRI signal response was observed within left dorsolateral PFC, inferior parietal lobule (IPL), and posterior insula. A negative relationship was observed between UCS expectancy and the threat-related response within dorsomedial PFC, ventromedial PFC, and anterior insula. Threat-related activity within dorsomedial PFC and left IPL varied with unconditioned SCR. Finally, anticipatory activity within the PFC, posterior cingulate, and amygdala showed an inverse relationship with threat-related activity within the brain regions that showed UCR diminution. The current findings suggest that the PFC and amygdala support learning-related processes that modulate the emotional response to a threat. 59 Key words: fMRI, learning, conditioning, unconditioned response, prefrontal cortex, emotion, fear, anxiety, skin conductance 60 Introduction Fear is considered an important defense mechanism due to its evolutionary role in survival [1–3]. The ability to form associations between a dangerous event and the cues that predict it allows an organism to better adapt to a changing environment [1,4]. An important aspect of this type of associative learning (i.e. Pavlovian fear conditioning) is that it allows an organism to more effectively avoid, escape, or minimize the impact of an impending threat [3–7]. Thus, from a functional perspective it is the response to the threat itself (i.e. unconditioned stimulus: UCS) that directly impacts survival and therefore may be the most biologically relevant feature of Pavlovian fear conditioning [4]. During Pavlovian fear conditioning, a neutral conditioned stimulus (CS) is paired with an aversive UCS. The conditioned response (CR) produced by the CS is typically used to index fear expression. For example, an increase in transient SCR expression during presentation of the CS. Traditionally, CR expression is taken as evidence that an association between the CS and UCS has been formed. In contrast, the unconditioned response (UCR) is generally considered an automatic, unlearned reaction to the aversive UCS. However, prior work has shown that unconditioned skin conductance responses (SCRs) diminish as associative learning develops during Pavlovian fear conditioning [8– 12]. For example, UCR amplitude is decreased to paired compared to unpaired presentations of the CS and UCS [9-14]. UCR diminution also appears to be mediated by conscious UCS expectancies [10,15–17]. For example, greater UCR diminution has been observed when participants expect a UCS compared to when the UCS is unexpected [10,15]. This phenomenon is generally referred to as conditioned UCR diminution. 61 A few brain imaging studies have investigated the neural correlates of conditioned UCR diminution [10,15,17,18]. These studies have demonstrated conditioned diminution of UCR activity within the dorsolateral PFC (dlPFC), dorsomedial PFC (dmPFC), ventromedial PFC (vmPFC), anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), inferior parietal lobule (IPL), anterior insula, and amygdala [10,15,17,18]. These findings are consistent with prior research that suggests the PFC regulates the emotional response [19,20]. This prior work indicates that the PFC projects to the amygdala and provides regulatory control over emotion-related processes during fear conditioning [1,19,21]. Further, the threat-related fMRI signal response within the PFC and amygdala influences the autonomic response (e.g. SCR) that is produced [10,15,17,22]. This process appears to be critical for normal, healthy emotional function. Converging lines of research indicate that healthy emotion regulation relies upon the PFC [19,20,23–25], and that anxiety disorders may be linked to insufficient regulatory control from the PFC. Further, PFC dysregulation is associated with increased amygdala reactivity [26–31] and an exaggerated emotional response to threats [11,32,33]. For example, prior work has shown that participants with low trait anxiety exhibit greater vmPFC activation compared to participants with high trait anxiety during cued fear conditioning [34]. In contrast, individuals with high trait anxiety showed a diminished vmPFC response that was associated with greater fear conditioned SCRs compared to participants with low trait anxiety [34]. Furthermore, our prior work has demonstrated that unconditioned fMRI signal responses from several brain regions fluctuate with individual differences in trait anxiety level [17]. Specifically, trait anxiety varied with dlPFC, dmPFC, PCC, and IPL activity such that, as anxiety level increased 62 the threat-related fMRI signal response within these brain regions increased [17]. Taken together, these studies suggest that anxiety level affects the magnitude of anticipatory and threat-related brain activation, which in turn influences the peripheral expression of emotion. Associative learning and expectancy processes are additional factors that influence the response produced by a threat. Prior work has demonstrated a decrease in the magnitude of brain activation once a cue-outcome relationship is established and predictable [35,36]. However, when an outcome violates expectations (i.e. surprise event) an increase in the magnitude of brain activity is observed [35,36]. Further, the magnitude of brain activation in response to an aversive stimulus is dependent upon the expectation of whether the aversive outcome is a certainty or only a possibility [15,38]. Prior work has demonstrated that the amplitude of the threat-elicited fMRI signal response within regions of the PFC, insula, cingulate, IPL, and amygdala varies with UCS expectancy [10,15,17,38]. More specifically, as UCS expectancy increases during CS presentations, the amplitude of the threat response decreases. These findings suggest that conditioned diminution of the UCR is in part mediated by UCS expectancies that are supported by regions of the PFC. In turn, these learning-related changes in the brain’s response to a threat appear to modify the peripheral emotional response that is expressed. For example, several studies have demonstrated that as UCS expectancy increases, the magnitude of unconditioned SCRs decrease [10,11,15]. Further, these findings parallel the learningrelated changes observed within the unconditioned fMRI signal response [10,15,17,38]. Taken together, these findings suggest that conscious expectation of an imminent threat may play an important role in modulation of the emotional response produced. However, 63 prior work has also demonstrated threat-elicited SCRs that did not diminish as expectation increased [17]. For example, unconditioned SCRs produced by a UCS that followed a CS− did not differ from SCRs to a UCS presented alone even though UCS expectancies differed between these conditions [17]. Prior work has also demonstrated greater UCR diminution to a UCS that followed a CS+ compared to a UCS presented after a CS− even when UCS expectancy ratings were equivalent [11]. In addition, diminished unconditioned SCRs have been observed to a UCS that followed a CS+ compared to a UCS that followed a CS− even after participants had been explicitly informed that the UCS would follow both CS presentations [12]. Taken together, these studies suggest that modulation of the threat-related emotional response is not solely mediated by conscious expectations. Instead, the findings suggest that associative learning processes independent of UCS expectancy also influence UCR diminution. Given that prior work suggests UCR diminution is in part mediated by expectancy independent processes, the present study was designed to better understand associative learning processes that influence UCR expression in the absence of differential UCS expectancies. The aim of this study was to determine the neural substrates that support expectancy-independent conditioned diminution of the threatrelated emotional response. Based on previous research, we expected a decrease in the unconditioned fMRI signal to develop with associative learning independent of UCS expectancy [11]. Given the importance of the amygdala, dlPFC, dmPFC, and vmPFC in the regulation and expression of emotion [1,19,20,38], we hypothesized that these brain regions would show UCR diminution during Pavlovian conditioning. Further, we expected activity within these brain regions to vary with individual differences in trait 64 anxiety. In turn, we expected amygdala activity to vary with the learning-related modulation of unconditioned SCRs [17,22,39–41]. Materials and Methods Participants: Twenty-one healthy right-handed volunteers participated in this study [8 male, 13 female; age = 23.05 ± 0.82 years (mean ± SEM); range = 19-34 years]. All participants were included in the UCS expectancy and fMRI data analyses. However, four non-responsive (SCR < 0.05 uSiemens) participants were excluded from SCR data analyses. Thus, a total of seventeen participants were included in the SCR analyses (7 male, 10 female; age = 23.59 ± 0.95 years; range = 19-34 years). Fifteen participants were included in the secondary fMRI data analyses that examined the relationship between behavior and brain activation (6 male, 9 female; age = 23.87 ± 1.06 years; range = 19-34 years). The six participants excluded from these secondary fMRI analyses consisted of the four participants with non-responsive SCR and two participants that did not complete the trait anxiety assessment. All subjects provided written informed consent in compliance with the University of Alabama at Birmingham Institutional Review Board. State-Trait Anxiety Inventory: Prior to the conditioning session, participants completed the State-Trait Anxiety Inventory (STAI; Form Y) for Adults [42]. The STAI is a selfassessment questionnaire that measures state and trait anxiety in terms of general negative affect [43]. The state scale reflects anxiety level at the current moment, whereas the trait scale reflects anxiety level experienced in general [42]. 65 Conditioned and unconditioned stimuli: Participants were exposed to a differential fear conditioning procedure in which the conditioned and unconditioned stimuli were presented through MR-compatible pneumatic headphones. Two tones (1025 and 1050 Hz; 10 s duration; 20 s ITI) that were difficult to discriminate served as the CSs. Our pilot work indicated that stimuli presented at these frequencies can be differentiated when presented back-to-back, but are difficult to discriminate when separated by a 20 s ITI. A loud (100db) white-noise served as the UCS (0.5 s duration). The UCS coterminated with one tone (CS+) and the second tone was presented alone (CS−) during the acquisition phase. The acquisition phase consisted of four 590 s blocks. A total of thirty-two trials of each CS were presented during the acquisition phase (8 trials of each CS were presented in each block). Additionally, each acquisition block contained one set of 3 test trials that consisted of UCS presentations that coterminated with the CS+ (CS+UCS) and CS− (CS−UCS), as well as presentations of the UCS alone (Figure 1). Thus, acquisition blocks consisted of 19 trials (8 CS+ & 8 CS− acquisition trials, as well as 1 CS+UCS, 1 CS−UCS, & 1 UCS alone test trial). Test trials during the acquisition blocks were presented on trials 17-19 of blocks 1 and 2; trials 13-15 of block 3; and trials 9-11 of block 4. The acquisition phase was followed by a 920 s test phase that consisted of 30 test trials (10 CS+UCS trials, 10 CS−UCS trials, 10 UCS alone trials). In total, there were 14 test trials for each stimulus (4 from the acquisition phase, 10 from the test phase). The 14 test trials were grouped into the first seven test trials (Early test trials) and the last seven test trials (Late test trials) for further analysis. The test trials were binned in this manner to evaluate learning-related changes in UCS expectancy in a manner consistent with our prior work using CS presentations that were easy to discriminate [17]. The stimuli were 66 counterbalanced and presented in a pseudorandom order such that no more than two trials of the same stimulus were consecutively presented. UCS expectancy: UCS expectancy was used to measure expectation of the UCS and assess whether the relationship between the CS and UCS had been learned. Using Presentation software (Neurobehavioral Systems, Inc.; Albany, CA), a UCS expectancy rating scale was presented on an IFIS-SA LCD (Invivo Corp.; Gainesville, FL) video screen located behind the subject's head and viewed through a mirror in front of the participant attached to the RF coil. An MRI compatible joystick (Current Designs; Philidelphia, PA) was used to monitor subjects’ expectancy of receiving the UCS. The joystick controlled a rating bar which was presented throughout the conditioning session on the video screen. Subjects were instructed to rate their UCS expectancy on a momentby-moment basis using a continuous scale from 0 to 100 (0 = certain the UCS would not be presented, 50 = uncertain whether the UCS would be presented, 100 = certain the UCS would be presented) to reflect their current UCS expectancy. UCS expectancy was calculated as the average response (1 s sample) at UCS onset. Additional details on this methodology have been published previously [44]. Skin conductance response: An MRI compatible physiological monitoring system (Biopac Systems; Goleta, CA) was used to collect SCR data as described in prior work [44]. SCR was sampled (2,000 Hz) with a pair of disposable radio-translucent electrodes (1cm diameter, Biopac Systems; Goleta, CA) from the distal phalanx of the middle and ring fingers of the nondominant hand. SCR data were processed using Biopac 67 AcqKnowledge 4.1 software. A 1 Hz low pass digital filter was applied and SCR data were resampled at 250 Hz. Unconditioned SCRs were limited to those that occurred within 10 s following the UCS presentation. Unconditioned SCRs smaller than 0.05 uSiemens were scored as 0. Functional MRI: Structural and functional imaging was completed on a 3 Tesla Siemens Allegra scanner. High-resolution anatomical images (MPRAGE) were obtained in the sagittal plane using a T1 weighted series (TR=2300 ms, TE=3.9 ms, flip angle=12⁰, FOV=25.6 cm, matrix=256 x 256, 160 slices, slice thickness1 mm, 0.5 mm gap) to serve as an anatomical reference. Blood oxygen level dependent fMRI of the entire brain was conducted using a gradient-echo echoplanar pulse sequence in an oblique-axial orientation (TR=2000 ms, TE=30 ms, flip angle=70º, FOV=24 cm, matrix=64 x 64, interleaved acquisition, 34 slices, slice thickness=4 mm, no gap) during each block of stimulus presentations. Functional image processing was performed with the Analysis of Functional NeuroImages (AFNI) software package [45]. Echo-planar time series data were corrected for slice timing offset, motion corrected, concatenated, reregistered to the fifth volume of the first imaging block, and spatially blurred using a 4 mm full-width-athalf-maximum Gaussian filter. Functional MRI data were analyzed at the individual subject level using the input from all stimuli in a multiple linear regression using a gamma variate hemodynamic response function. Regressors to account for brain activity not related to the UCR on test trials included reference waveforms for the CS+ and CS− during acquisition, UCS during acquisition, CS+ and CS− on test trials, joystick movement, and head motion parameters. 68 The regressors of interest for this study modeled the unconditioned fMRI signal response to UCS presentations during each type of test trial (i.e. CS+UCS, CS−UCS, and the UCS alone). Separate reference waveforms were used for Early and Late test trials in this analysis. Percent signal change on test trials was used as an index of the magnitude of the unconditioned fMRI signal response produced by the UCS. Functional maps reflecting percent signal change were converted to the Talairach and Tournoux stereotaxic coordinate system for group analyses [46]. Based on prior work [10,15,17], group level analyses were restricted using an anatomical mask, to the PFC, cingulate cortex, IPL, insula, and amygdala to reduce the number of voxel-wise comparisons. We conducted a repeated-measures ANOVA to test for a main effect of stimulus (CS+UCS, CS−UCS, and UCS alone) and trial (Early vs. Late test trials), as well as a stimulus x trial interaction. A voxel-wise threshold of p < 0.05 (corrected) was employed by using an uncorrected threshold of p < 0.005 and a cluster volume larger than 510 mm3 (9 voxels of 3.75 x 3.75 x 4.00 mm dimension). These threshold criteria were based on Monte Carlo simulations that were used to reject smaller clusters of activation produced by chance alone (false positives) [47,48]. Followup t-test comparisons were conducted in SPSS on the mean percent signal change activation passing the significance threshold (p < 0.05 Bonferonni corrected) for the ANOVA. Two different analysis procedures (i.e. correlation and multiple linear regression) were completed to investigate the relationship between our behavioral measures (i.e. trait anxiety, UCS expectancy, and SCR) and the unconditioned fMRI signal response from brain regions that demonstrated learning-related changes in the ANOVA (i.e. functional 69 regions of interest; ROI). Although these analyses are similar there are important differences (see [17] for additional discussion). In short, separate correlation analyses assessed the relationship between each of our behavioral measures and the mean percent signal change within an ROI as a whole, while the regression analysis evaluated these relationships on a voxel-wise basis. The four participants without measurable SCR data and the two participants without a trait anxiety score were excluded from this regression analysis because there were no data points to include in the model. AlphaSim [45,47] was used to conduct Monte Carlo simulations limited to the functional ROI from our repeated-measures ANOVA that demonstrated a main effect of stimulus or stimulus x trial interaction. A voxel-wise threshold of p < 0.005 and a cluster volume larger than 225mm3 (4 voxels of 3.75 x 3.75 x 4.00mm dimension) was employed, resulting in a FWE corrected significance threshold of p < 0.05. Prior work has demonstrated a relationship between the magnitude of the fMRI signal response within the amygdala and SCR production during Pavlovian fear conditioning [10,17,22,39–41]. Therefore an anatomical mask was employed to include the amygdala in the group level regression analysis. Prior work suggests that the PFC and amygdala produce and regulate the emotional response to aversive stimuli [17, 19,38]. Therefore, we completed an additional voxel-wise multiple regression analysis to identify anticipatory PFC, cingulate, and amygdala activity that varied with the unconditioned fMRI signal response obtained from the functional ROI from our ANOVA. This analysis was restricted to CS+UCS and CS−UCS trials because a CS was not presented during UCS alone trials to elicit an anticipatory response. This analysis included a regressor representing trial type (CS+UCS 70 & CS−UCS), a regressor for the amplitude of the unconditioned fMRI signal response from our functional ROI, and a regressor for the interaction of trial type and unconditioned fMRI signal response amplitude. As indicated by Monte Carlo simulations, a voxel-wise threshold of p < 0.005 and a cluster volume larger than 510 mm3 (9 voxels of 3.75 x 3.75 x 4.00 mm dimension) was employed resulting in a FWE corrected significance threshold of p < 0.05. Given our a priori hypotheses and the relatively small volume of the amygdala, we used a voxel-wise threshold of p < 0.005 and a cluster volume larger than 112 mm3 (2 voxels of 3.75 x 3.75 x 4.00 mm dimension) for this area (p < 0.05 corrected). Results UCS expectancy: Repeated measures ANOVA revealed significant differences in UCS expectancy during the test trials. Results showed a main effect for stimulus type (F[1,20] = 38.30, p < 0.05) and a main effect for trial (F[1,20] = 33.68, p < 0.05). There was no stimulus by trial interaction (F < 1.00). UCS expectancy was greater during Early test trials on CS+UCS [mean ± SEM (adjusted for between subject variance [49]): 65.85 ± 2.86; t[20] = 5.55, p < 0.05] and CS−UCS (70.60 ± 3.97; t[20] = 5.48, p < 0.05) than on UCS alone trials (34.31 ± 3.89). UCS expectancy did not differ for CS+UCS and CS−UCS (t[20] = -1.00) presentations during Early test trials. UCS expectancy was greater during Late test trials on CS+UCS (86.09 ± 2.84; t[20] = 6.20, p < 0.05) and CS−UCS (91.83 ± 2.97; t[20] = 6.29, p < 0.05) compared to UCS alone (48.58 ± 4.56) trials. During the Late test trials, UCS expectancy was also greater during CS−UCS presentations than on CS+UCS presentations (t[20] = -2.31, p < 0.05) (Figure 2a). 71 Skin conductance response: Repeated measures ANOVA also revealed significant differences in unconditioned SCR amplitude during the test trials. There was a main effect for stimulus type (F[1,16] = 4.85, p < 0.05). There was also a trend for a main effect of trial (F[1,16] = 4.44, p = 0.051), and a trend for a stimulus by trial interaction (F[1,16] = 4.39, p = 0.052). T-test comparisons revealed a significantly diminished unconditioned SCR for CS+UCS (1.07 ± 0.08; t[16] = -3.05, p < 0.05) and CS−UCS trials (1.38 ± 0.17; t[16] = -2.04, p < 0.05) compared to the UCS alone (1.88 ± 0.22) during Early test trials. There was not a significant difference in unconditioned SCR between CS+UCS and CS−UCS trials (t[16] = -1.49) during Early test trials. Also, there was not a significant difference in unconditioned SCR between CS+UCS trials (0.98 ± 0.20) and CS−UCS trials (0.90 ± 0.18; t < 1.00) during Late test trials. Unconditioned SCR during CS+UCS (t[16] = -1.08) and CS−UCS (t[16] = -1.38) trials also did not differ from UCS alone trials (1.30 ± 0.15) during the Late test trials (Figure 2b). Functional MRI: Repeated measures ANOVA revealed significant differences in the magnitude of the unconditioned fMRI signal response within several brain regions (Tables 1 and 2; Figure 3). UCR diminution was observed within the dlPFC, dmPFC, vmPFC, and anterior insula replicating prior work [10,15,17] (Table 1; Figure 3). We also observed potentiation of the UCR within several brain regions including the dlPFC, IPL, and posterior insula (Table 2). Within each of these brain regions the unconditioned fMRI signal response demonstrated a main effect for stimulus type (F[20] > 6.06; p < 0.05 corrected). A main effect for trial (i.e. Early vs. Late test trials) was observed within 72 the dlPFC, IPL, and anterior insula (Table 3). A stimulus x trial interaction was observed within left IPL (Table 2). T-test comparisons were completed on the mean fMRI signal from each volume of activation that passed the significance threshold (p < 0.05 corrected) for the main effect of stimulus type. There was no difference in the unconditioned fMRI signal response for CS+UCS compared to CS−UCS trials within the functional ROIs identified by the ANOVA. The unconditioned fMRI signal response was diminished for the CS+UCS and CS−UCS compared to the UCS alone within left dlPFC, dmPFC, vmPFC, and left anterior insula (Table 1). However, greater activation was observed for CS+UCS and CS−UCS trials compared to UCS alone trials within left dlPFC and bilateral IPL. Additionally, greater activation for CS−UCS vs. UCS alone trials was observed within bilateral posterior insula (Table 2). A correlation analysis was performed on the mean percent signal change from each of the functional ROIs from the ANOVA and trait anxiety, UCS expectancy, and unconditioned SCR amplitude measures (Tables 1 and 2; p < 0.05 Bonferroni corrected). The percent signal change within these brain regions did not vary with trait anxiety or unconditioned SCR. These findings replicate prior work that used CS presentations that were easy to discriminate during Pavlovian fear conditioning [17]. However, there was a significant correlation observed between UCS expectancy ratings and activity within dmPFC, vmPFC, left IPL, and left anterior insula (Tables 1 and 2), generally consistent with prior research [17]. A voxel-wise multiple linear regression analysis was conducted to evaluate brain activity within the functional ROI that varied with individual differences in behavior. The amygdala was also included in this analysis because prior work suggests this brain region 73 mediates learning-related changes in SCR production [22,39–41]. The regression model accounted for stimulus type, trait anxiety, UCS expectancy, and unconditioned SCR. This analysis demonstrated that unconditioned SCR amplitude explained unique variability in the activation observed within the left dmPFC (r = .34; Talairach coordinates: -7.5, -16.3, 50; volume: 280 mm3) and left IPL (r = .28; Talairach coordinates: -41.5, -36.2, 42.3; volume: 237 mm3), but not within the amygdala. There were no brain regions within the functional ROI that varied with UCS expectancy or trait anxiety. We also conducted a group level regression analysis to investigate whether anticipatory activation (i.e. the CR) within the PFC, cingulate, and amygdala varied with threat-related activity (i.e. the UCR) from brain regions that demonstrated UCR diminution (Table 1). Anticipatory activity within dlPFC, dmPFC, vmPFC, ventrolateral PFC (vlPFC), posterior cingulate, and amygdala showed a negative relationship with the threat-related fMRI signal response (i.e. UCR) within many of the brain regions in which conditioned UCR diminution was observed (Table 4). Anticipatory activation within left vlPFC was negatively correlated with the threat response within each of the brain areas that showed UCR diminution. This effect was also observed between anticipatory activation within the right vlPFC and threat-related activity within each of the functional ROI that showed UCR diminution, except for the left dlPFC (Table 4). A negative relationship was also observed between anticipatory activity within vmPFC and the threat-related response within left dlPFC, dmPFC, and left anterior insula (Figure 4a and c). A similar pattern was observed between anticipatory activation within dlPFC, dmPFC, vlPFC, and PCC and the threat-related response within vmPFC (Figure 4e). Finally, 74 anticipatory amygdala activity showed a similar negative relationship with the threatrelated response within dmPFC and left anterior insula (Figure 4b and d). Discussion Learning the relationship between a threat and the cues that predict it is critical to survival. These cue-threat relationships are established when there is a discrepancy between expectation and outcome [48], by associative learning and expectancy-related processes that may somewhat independently influence the magnitude of the threatelicited response [10–12,15,17]. Therefore, the present study used CS presentations that were difficult to discriminate to investigate the neural processes that support learningrelated changes in the UCR in the absence of differential UCS expectancies to better understand associative learning processes that mediate UCR diminution. Learning-related differences in UCS expectancy and unconditioned SCR expression were observed in the present study. By design, UCS expectancy ratings were high on CS+UCS and CS−UCS trials. In contrast, UCS expectancy on UCS alone trials were rated around 50 (Figure 2a). These findings demonstrate that participants expected the UCS following the CS+ and CS−, but remained uncertain about the timing of the UCS alone during the conditioning session. UCS expectancy was also associated with the amplitude of unconditioned SCR expression. Specifically, unconditioned SCR amplitude was diminished on CS+UCS and CS−UCS trials (when UCS expectancy was high) compared to the UCS alone (when UCS expectancy was lower) on Early test trials (Figure 2b). These findings are consistent with prior work that has shown a decreased UCR when the UCS is predictable vs. unpredictable [8,9,11,49]. Based on our pilot data, 75 we also expected to observe greater unconditioned SCR diminution during CS+UCS than CS−UCS trials in this study. Findings of differential SCR, with equivalent UCS expectancy, would allow us to address questions related to learning independent of conscious expectations. However, no difference in unconditioned SCR expression was observed between CS+UCS and CS−UCS trials. Therefore, there is no evidence of conditioned UCR diminution that is independent of UCS expectancy in the present study. The lack of differential SCRs on CS+UCS vs. CS−UCS trials is likely due to the lack of discriminative control gained by the CS. Given that the CS+ and CS− were difficult to discriminate, evidenced by high expectancy ratings, participants appear to have interpreted the acquisition phase as a 50% reinforcement schedule of a single CS rather than separate presentations of a CS+ and CS−. Therefore, the lack of differential unconditioned SCRs during CS+UCS compared to CS−UCS trials appears to be due to a deficit in learning the CS discrimination. Although there is no evidence of learning, independent of expectancy, we did observe conditioned UCR diminution in other contrasts in the present study. Unconditioned SCR amplitude was diminished on CS+UCS and CS−UCS trials compared to UCS alone trials. This response pattern was also observed in the fMRI signal within the left dlPFC (z = 35.0), dmPFC, vmPFC, and left anterior insula. These findings are generally consistent with prior studies that have demonstrated UCR diminution within these brain regions [10,15,17]. However, contrary to our prior work, an enhanced threat-related fMRI response was observed within other brain regions including a more superior region of left dlPFC (z = 51.7), bilateral IPL, and bilateral posterior insula. Similar to the SCR data, there were no differences between CS+UCS and 76 CS−UCS trials within any of the brain regions that demonstrated learning-related changes in the unconditioned fMRI signal response (Tables 1 and 2). Taken together, the present findings replicate prior work that has shown learning-related changes in brain activation that resemble the pattern of the emotional response produced [15,17,22,39,40]. Prior work suggests that UCS expectancy modulates the amplitude of the threatrelated fMRI signal response [10,15,17]. Prior work has also shown that conscious expectation influences the emotional response evoked by a threat [10,11,15–17]. Similar results were also observed in the current study. Consistent with our prior work, a negative relationship was observed between UCS expectancy and brain activity in regions that showed UCR diminution [17]. Specifically, a negative relationship was observed between UCS expectancy and brain activation within dmPFC, vmPFC, and left anterior insula (Table 1). However, a positive relationship was observed between UCS expectancy and the magnitude of the threat-related fMRI signal response within left IPL (Table 2). This relationship between the unconditioned fMRI signal response and UCS expectancy was not observed in our group level multiple linear regression analysis. The regression analysis was conducted on a voxel-wise basis and accounted for additional measures of interest (e.g. stimulus type). These findings suggest that UCS expectancy varied with the mean percent signal change of the unconditioned fMRI signal response within the ROIs as a whole. However, UCS expectancy did not explain unique variance within the unconditioned fMRI signal response within these brain regions. These findings replicate prior conditioning work that employed CS presentations that were easy to discriminate [17]. In addition, our group level regression analysis revealed that the amplitude of the unconditioned SCR explained unique variance in the fMRI data. Specifically, a positive 77 relationship was observed between threat-related SCR production and brain activation within the dmPFC and left IPL. Taken together these findings support previous research that suggests that regions of the PFC support associative learning processes [51,52], and affect the peripheral emotional response [19–21]. The PFC appears to support top-down processes that are important for emotion regulation. Prior work has demonstrated a negative relationship between anticipatory PFC activity and the threat-related response within the amygdala [17,38]. Specifically, as anticipatory PFC activity increases, the neural response to the threat decreases [17]. In turn, the threat-elicited fMRI signal appears to mediate the learning-related changes observed in the peripheral emotional response to a threat [11,15,17]. The data from the current study are generally consistent with this prior work. In the present study, a negative relationship was observed between anticipatory activation within the PFC and threat-related activity within brain regions that showed UCR diminution (Table 1). Specifically, as anticipatory activity within dlPFC, dmPFC, vlPFC, vmPFC, and the amygdala increased the threat-related response within left dlPFC, dmPFC, vmPFC, and anterior insula decreased (Table 4, Figure 4). Further, activity within dmPFC and left IPL was positively correlated with threat-related SCR expression. These findings demonstrate that anticipatory brain activity affects the response elicited by a threat, which in turn affects the emotional response. In summary, conditioned diminution of the unconditioned SCR and fMRI signal response was observed during Pavlovian fear conditioning. UCR diminution was observed within left dlPFC, dmPFC, vmPFC, and left anterior insula. Consistent with prior work, many of the brain regions that showed learning-related changes in the 78 unconditioned fMRI signal response varied with UCS expectancy and unconditioned SCR production [10,15,17]. Further, a negative relationship was observed between the brain regions that showed UCR diminution and anticipatory PFC activiation (i.e. dlPFC, dmPFC, vlPFC, and vmPFC). This finding supports prior work that suggests top-down mechanisms provided by the PFC inhibit the emotional response evoked by a threat. The findings from the current study provide a better understanding of the neural mechanisms that support associative learning processes that modulate the threat-related response. Acknowledgements: This research was supported by the University of Alabama at Birmingham Faculty Development Grant Program and NIH R01 MH098348 (DCK). 79 References 1. Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: A critical review. Neuroscience & Biobehavioral Reviews. 2006;30(2):188–202. 2. LeDoux J. The amygdala. Current biology: CB. 2007;17(20):868–74. 3. Mineka S, Öhman A. Born to fear: non-associative vs associative factors in the etiology of phobias. Behaviour research and therapy. 2002 Feb;40(2):173–84. 4. Domjan M. Pavlovian conditioning: a functional perspective. Annual review of psychology. 2005 Jan;56:179–206. 5. Franchina JJ. Escape behavior and shock intensity: within subject versus betweengroups comparisons. Journal of comparative and physiological psychology. 1969;69(2):241–5. 6. Helmstetter FJ, Bellgowan PS. Lesions of the amygdala block conditional hypoalgesia on the tail flick test. Brain research. 1993;612(1-2):253–7. 7. Kamin LJ. Traumatic avoidance learning: the effects of CS−US interval with a trace-conditioning procedure. Journal of comparative and physiological psychology. 1954;47(1):65–72. 8. Baxter R. Diminution and recovery of the UCR in delayed and trace classical GSR conditioning. Journal of experimental psychology. 1966;71(3):447–51. 9. Kimmel E. Judgments of UCS intensity and diminution of the UCR in classical GSR conditioning. Journal of experimental psychology. 1967;73:532–43. 10. Knight DC, Waters NS, King MK, Bandettini PA. Learning-related diminution of unconditioned SCR and fMRI signal responses. NeuroImage. Elsevier B.V.; 2010 Jan 1;49(1):843–8. 11. Knight DC, Lewis EP, Wood KH. Conditioned diminution of the unconditioned skin conductance response. Behavioral neuroscience. 2011 Aug;125(4):626–31. 12. Marcos JL, Redondo J. Effects of conditioned stimulus presentation on diminution of the unconditioned response in aversive classical conditioning. Biological psychology. 1999 Jun;50(2):89–102. 13. Lykken DT, Macindoe I, Tellegen A. Preception: Autonomic Response to Shock as a Function of Predictability in Time and Locus. Psychophysiology. 1972;9(3):318–33. 80 14. Peeke SC, Grings WW. Magnitude of UCR as a function of variability in the CS– UCS relationship. Journal of experimental psychology. 1968;77:1–6. 15. Dunsmoor JE, Bandettini PA, Knight DC. Neural correlates of unconditioned response diminution during Pavlovian conditioning. NeuroImage. 2008 Apr 1;40(2):811–7. 16. Rust J. Unconditioned response diminution in the skin resistance response. The Journal of General Psychology. 1976;95:77–84. 17. Wood KH, Ver Hoef LW, Knight DC. Neural mechanisms underlying the conditioned diminution of the unconditioned fear response. NeuroImage. Elsevier Inc.; 2012 Mar;60(1):787–99. 18. Linnman C, Rougemont-Bücking A, Beucke JC, Zeffiro TA, Milad MR. Unconditioned responses and functional fear networks in human classical conditioning. Behavioural brain research. Elsevier B.V.; 2011 Aug 1;221(1):237– 45. 19. Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008 Sep 11;59(5):829–38. 20. Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. Journal of cognitive neuroscience. 2002;14:1215–29. 21. Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behavioral neuroscience. 2004 Apr;118(2):389–94. 22. Knight DC, Nguyen HT, Bandettini PA. The role of the human amygdala in the production of conditioned fear responses. NeuroImage. 2005 Jul 15;26(4):1193– 200. 23. Basten U, Stelzel C, Fiebach CJ. Trait Anxiety Modulates the Neural Efficiency of Inhibitory Control. Journal of cognitive neuroscience. 2011;(10):3132–45. 24. Klumpp H, Ho SS, Taylor SF, Phan KL, Abelson JL, Liberzon I. Trait anxiety modulates anterior cingulate activation to threat interference. Depression and anxiety. 2011 Mar;28(3):194–201. 25. Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. NeuroImage. 2006 Jan 1;29(1):106–16. 81 26. Etkin A, Wager TD. Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. American Journal of Psychiatry. 2007;164(October):1476–88. 27. Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biological psychology. 2006 Jul;73(1):61–71. 28. Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiologial basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry. 2009;66(12):1075–82. 29. Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biological psychiatry. 2006 Aug 15;60(4):376–82. 30. Sehlmeyer C, Dannlowski U, Schöning S, Kugel H, Pyka M, Pfleiderer B, et al. Neural correlates of trait anxiety in fear extinction. Psychological medicine. 2011 Apr;41(4):789–98. 31. Somerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: Correlations with state anxiety. Biological psychiatry. 2004 May 1;55(9):897–903. 32. Cook EW, Davis TL, Hawk LW, Spence EL, Gautier CH. Fearfulness and startle potentiation during aversive visual stimuli. Psychophysiology. 1992 Nov;29(6):633–45. 33. Grillon C, Ameli R, Goddard A, Woods SW, Davis M. Baseline and fearpotentiated startle in panic disorder patients. Biological psychiatry. 1994;35:431– 9. 34. Indovina I, Robbins TW, Núñez-Elizalde AO, Dunn BD, Bishop SJ. Fearconditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron. 2011 Feb 10;69(3):563–71. 35. Casey BJ, Thomas KM, Welsh TF, Badgaiyan RD, Eccard CH, Jennings JR, et al. Dissociation of response conflict, attentional selection, and expectancy with functional magnetic resonance imaging. Proceedings of the National Academy of Sciences of the United States of America. 2000 Jul 18;97(15):8728–33. 36. Fletcher PC, Anderson JM, Shanks DR, Honey R, Carpenter TA, Donovan T, et al. Responses of human frontal cortex to surprising events are predicted by formal associative learning theory. Nature neuroscience. 2001 Oct;4(10):1043–8. 82 37. Dunsmoor JE, Bandettini PA, Knight DC. Impact of continuous versus intermittent CS-UCS pairing on human brain activation during Pavlovian fear conditioning. Behavioral neuroscience. 2007 Aug;121(4):635–42. 38. Sarinopoulos I, Grupe DW, Mackiewicz KL, Herrington JD, Lor M, Steege EE, et al. Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cerebral cortex. 2010 Apr;20(4):929–40. 39. Cheng DT, Knight DC, Smith CN, Stein EA, Helmstetter FJ. Functional MRI of human amygdala activity during Pavlovian fear conditioning: Stimulus processing versus response expression. Behavioral Neuroscience. 2003;117(1):3–10. 40. Cheng DT, Knight DC, Smith CN, Helmstetter FJ. Human amygdala activity during the expression of fear responses. Behavioral neuroscience. 2006 Dec;120(6):1187–95. 41. Cheng DT, Richards J, Helmstetter FJ. Activity in the human amygdala corresponds to early, rather than late period autonomic responses to a signal for shock. Learning & Memory. 2007;14(7):485–90. 42. Spielberger C. State–Trait Anxiety Inventory for Adults. Redwood City, CA: Mind Garden; 1983. 43. Grös DF, Antony MM, Simms LJ, McCabe RE. Psychometric properties of the State-Trait Inventory for Cognitive and Somatic Anxiety (STICSA): Comparison to the State-Trait Anxiety Inventory (STAI). Psychological assessment. 2007 Dec;19(4):369–81. 44. Knight DC, Wood KH. Investigating the neural mechanisms of aware and unaware fear memory with FMRI. Journal of visualized experiments: JoVE. 2011 Jan;(56):1–6. 45. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and biomedical research, an international journal. 1996 Jun;29(3):162–73. 46. Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. 47. Forman S, Cohen J, Fitzgerald M, Eddy W, Mintun M, Noll D. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic resonance in medicine. 1995;33(5):636–47. 83 48. Saad ZS, Chen G, Reynolds RC, Christidis PP, Hammett KR, Bellgowan PSF, et al. Functional imaging analysis contest (FIAC) analysis according to AFNI and SUMA. Human brain mapping. 2006 May;27(5):417–24. 49. Loftus GR, Masson MEJ. Using confidence intervals in within-subject designs. Psychonomic Bulletin & Review. 1994 Dec;1(4):476–90. 50. Rescorla RA. Behavioral studies of Pavlovian conditioning. Annual review of neuroscience. 1988;11:329–52. 51. Orlebeke JF, Van Doornen LJ. Preception (UCR diminution) in normal and neurotic subjects. Biological psychology. 1977 Mar;5(1):15–22. 52. Knight DC, Smith CN, Cheng DT, Stein EA, Helmstetter FJ. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cognitive, affective & behavioral neuroscience. 2004 Sep;4(3):317–25. 53. Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biological Psychiatry. 2003 Sep;54(5):504–14. 84 Table 1. Regions showing conditioned diminution of the UCR. Region Vol (mm3) Talairach coordinates x y z CS+UCS vs. CS−UCS CS−UCS vs. UCS alone CS+UCS vs. UCS alone t t t Trait r SCR r UCS Expectancy r Dorsolateral PFC 3739 −46.3 10.8 35.0 n.s. −4.23 −3.81 0.04 −0.04 −0.17 Dorsomedial PFC 5670 −1.6 24.5 45.9 n.s. −4.52 −4.18 0.04 −0.04 −0.36* Ventromedial PFC 515 −0.9 52.7 −0.5 n.s. −4.33 −3.96 −0.02 0.05 −0.50* Left Anterior Insula Left 1046 −34.6 16.3 1.7 n.s. −3.73 −3.99 0.06 0.18 −0.48* Note. Location, volumes, and coordinates from Talairach and Tournoux (1988) for the center of mass for areas of activation. Significance criteria: ANOVA F[20] > 6.06, p < 0.05 (corrected); t[20] p < 0.05 (corrected). Significance criteria for two-tailed correlations: * indicates p < 0.05 (corrected). 85 Table 2. Regions showing potentiation of the UCR. Region Vol (mm3) Talairach coordinates x y z CS+UCS vs. CS−UCS CS−UCS vs. UCS alone CS+UCS vs. UCS alone t t t Trait r SCR r UCS Expectancy r Main effect of stimulus type Dorsolateral PFC 1286 −7.3 −14.8 51.7 n.s. 3.93 3.57 0.15 0.14 0.30 Right 954 56.3 −29.3 28.4 n.s. 3.82 3.45 0.32 −0.06 0.28 Left 2618 −39.2 −40.9 51.0 n.s. 3.85 3.44 0.15 −0.07 0.43* Left 1054 −60.9 −27.0 27.7 n.s. 4.20 3.39 0.28 −0.01 0.34 Right 3155 41.1 −7.7 10.8 n.s. 5.62 n.s. 0.16 −0.02 0.28 Left 2106 −41.9 −14.2 12.6 n.s. 4.29 n.s. 0.22 −0.12 0.31 −42.8 −34.4 38.7 n.s. n.s. 4.11 0.14 −0.06 Left Inf. Parietal Lobule Posterior Insula Stimulus x trial interaction Inf. Parietal Lobule Early test trials 577 0.33 Late test trials n.s. n.s. n.s. 0.05 0.09 0.20 Note. Location, volumes, and coordinates from Talairach and Tournoux (1988) for the center of mass for areas of activation. Significance criteria: ANOVA F[20] > 6.06, p < 0.05 (corrected); t[20] p < 0.05 (corrected). Significance criteria for two-tailed correlations: * indicates p < 0.05 (corrected). 86 Table 3. Regions showing change over time. Talairach coordinates Region Dorsolateral PFC Inf. Parietal Lobule Hemisphere 3 Vol (mm ) x y z Right 1072 23.7 44.0 32.0 Left 591 -45.8 9.0 29.5 Right 553 51.0 -41.9 24.7 Anterior Insula Left 1258 -44.4 21.0 0.9 Note. Location, volumes, and coordinates from Talairach and Tournoux (1988) for the center of mass for areas of activation. Significance criteria: F[20] > 10.00; p < 0.05 (corrected). 87 Table 4. Regions showing a relationship between anticipatory and threat-related activity. UCR DIMINUTION FUNCTIONAL ROIs Talairach Vol coordinates Region Dorsolateral PFC Left 3 (mm ) x y ANTICIPATORY BRAIN ACTIVATION Vol z ↔ 3739 −46.3 10.8 35.0 3 Region Ventrolateral PFC (mm ) x 817 −37.4 29.9 −10.4 665 5.1 50.7 −9.0 536 32.8 42.6 15.4 Right 1997 35.2 21.3 −8.2 Right 1016 20.9 60.6 1.6 1483 −37.0 25.8 −9.1 601 1.4 51.2 −4.7 149 22.4 −1.7 −17.8 10761 −1.1 24.1 33.9 Right 3911 35.2 33.8 29.5 Right 2593 46.4 7.9 30.6 Right 663 34.8 −3.6 48.2 Left 2993 −33.4 26.8 34.8 Left 1513 −49.8 9.0 30.0 Left 555 −24.5 13.8 50.9 Left Ventromedial PFC Dorsomedial PFC 5670 −1.6 24.5 45.9 ↔ Talairach coordinates y z Dorsolateral PFC Right Ventrolateral PFC Left a Ventromedial PFC Amygdala Rightb Ventromedial PFC 515 −0.9 52.7 −0.5 ↔ Dorsomedial PFCe,f Dorsolateral PFC Ventrolateral PFC Right 1906 41.1 32.2 7.2 Left 1523 −43.3 30.6 3.1 5898 −3.0 −32.8 32.9 Left 983 −37.2 21.8 −9.7 Right 1994 36.4 22.3 −8.1 975 2.3 50.5 −3.6 Posterior cingulate ↔ Anterior Insula Left 1046 −34.6 16.3 1.7 e Ventrolateral PFC c Ventromedial PFC Amygdala Rightd 182 23.9 −1.2 −15.8 Note. Location, volumes, and coordinates from Talairach and Tournoux (1988) for the center of mass for areas of activation. Significance criteria: t[20] > 2.98, p < 0.05 (corrected). The UCR amplitude (from ROI on left side of table) varied with the anticipatory response (i.e. the CR) within the dorsolateral, dorsomedial PFC, ventromedial PFC, and posterior cingulate (right side of the table). Exploratory analysis of amygdala and hippocampal activation small volume correction applied t[20] > 2.98, 112 mm3, p < 0.05 (corrected) (right side of the table). Letters (a-f) correspond to images and graphical representation presented in Figure 4. 88 Acquisition Phase Test Phase CS+ CS+ UCS UCS CS- CS- UCS UCS 0 10 20 30 Time (s) 40 50 No CS UCS 0 10 20 30 Time (s) 40 50 Figure 1. Conditioned and unconditioned stimuli. The acquisition blocks consisted of CS+ (8 trials), CS− (8 trials), and test trials (1 CS+UCS, 1 CS−UCS, and 1 UCS alone trial). The acquisition phase consisted of four acquisition blocks, followed by the test phase. The test phase consisted of 10 CS+UCS trials, 10 CS−UCS trials, and 10 UCS alone trials. Stimuli were counterbalanced and presented in a pseudorandom order such that no more than two trials of the same stimulus were consecutively presented. 89 UCS Expectancy 100 a 80 60 40 CS+UCS CS-UCS UCS alone 20 Unconditioned SCR 2.5 b 2.0 1.5 1.0 0.5 1-7 8-14 Trials Figure 2. UCS expectancy and unconditioned SCR. a) Learning-related differences in UCS expectancy. UCS expectancy on Early and Late test trials (1–7) was higher during CS+UCS and CS−UCS trials vs. UCS alone trials. b) Learning-related changes in unconditioned SCR expression were also observed. During Early test trials unconditioned SCRs were diminished on CS+UCS and CS−UCS trials compared to UCS alone trials. No differences were observed between CS+UCS and CS−UCS trials. During Late test trials no differences were observed between CS+UCS, CS−UCS, or UCS alone trials. 90 0.5 Dorsolateral PFC * fMRI signal (% ) fMRI signal (% ) Dorsomedial PFC * 0.4 0.3 0.2 0.1 CS+UCS CS-UCS * 0.4 * 0.3 0.2 0.1 0.0 UCS alone CS+UCS CS-UCS UCS alone z = 38 * 0.2 Anterior Insula * fMRI signal (% ) fMRI signal (% ) Ventromedial PFC 0.1 0.0 -0.1 -0.2 CS+UCS CS-UCS UCS alone Right Left * 0.5 * 0.4 0.3 0.2 0.1 CS+UCS CS-UCS UCS alone z = -2 Figure 3. UCR diminution within the fMRI signal response. Significant diminution of the unconditioned fMRI signal response was observed within the prefrontal cortex (PFC) and anterior insula during test trials. UCR amplitude within these brain regions was reduced when the UCS followed the CS+ (i.e. CS+UCS trials) and CS− (i.e. CS−UCS trials) compared to when the UCS was presented alone. There was no difference on CS+UCS versus CS−UCS trials. Graphs reflect the mean amplitude (% signal change) of all voxels within volumes of activation. Error bars reflect SEM after adjusting for between-subject variance [49]. Asterisk indicates significant difference. 91 Relationship between Anticipatory and Threat-related Activity vmPFC and R. Amyg CR ↔ dmPFC UCR r = −.60; p < 0.05 r = −.53; p < 0.05 a b x=2 y = -3 vmPFC and R. Amyg CR ↔ Ant. Insula UCR r = −.66; p < 0.05 r = −.58; p < 0.05 c d x=2 y = -2 dmPFC and PCC CR ↔ vmPFC UCR r = −.55; p < 0.05 r = −.57; p < 0.05 f 0.2 vmPFC UCR e x=2 0.0 -0.2 -0.4 CS+UCS CS-UCS -0.4 -0.2 0.0 0.2 0.4 dmPFC CR Figure 4. Relationship between anticipatory and threat-related activity. Threat-related activity, extracted from the ROI depicted in Table 1 and Figure 3, was included in a regression analysis to investigate differences in the relationship between anticipatory activity (i.e. CR) and threat-related activity (i.e. UCR) on CS+UCS and CS−UCS trials. A negative relationship between anticipatory and threat-related activity (% signal change) was observed in several areas of the PFC, cingulate, and amygdala (a-e). Correlation values comparing the anticipatory and threat-related response within these brain areas are presented above the brain images. The correlation value above image (e) represents activation observed between PCC CR and vmPFC UCS. The correlation value for dmPFC CR and vmPFC UCR is presented in graph (f). Talairach coordinates for the depicted areas of activation are presented in Table 4 and labeled with letters (a-e) corresponding to each image above. 92 93 CONTROLLABILITY AND PREDICTABILITY DIMINISH THE NEURAL RESPONSE TO A THREAT KIMBERLY H. WOOD, KENTON H. BOWEN, JOSHUA R. SHUMEN, MURIAH D. WHEELOCK, LAWRENCE W. VER HOEF, AND DAVID C. KNIGHT In preparation for Journal of Neuroscience Format adapted for dissertation Abstract The ability to predict and control stressful events influences our emotional response to future stressors. Prior animal research has demonstrated a diminished emotional response to predictable and controllable stressors, whereas unpredictable and uncontrollable stressors result in an enhanced emotional response. The present study was designed to better understand the effect of predictability and controllability on the threat-related emotional response. Two groups of healthy volunteers participated in a Pavlovian fear conditioning study during functional magnetic resonance imaging (fMRI). Similar to prior animal research, the groups consisted of yoked pairs where one group (Controllable Condition; CC) was able to terminate the unconditioned stimulus (UCS), and the other group (Uncontrollable Condition; UC) was not able to terminate the UCS. We also assessed the influence of state anxiety and UCS expectancy on the modulation of threatrelated skin conductance response (SCR), startle eye-blink electromyography (EMG), and fMRI signal response. The threat-related fMRI signal response was diminished on predictable compared to unpredictable trials within the dorsolateral prefrontal cortex (PFC), dorsomedial PFC, ventromedial PFC, ventrolateral PFC, and posterior cingulate for both CC and UC groups. A predictability x controllability interaction was observed within ventromedial PFC and left hippocampus. Specifically, the threat-related response within these brain regions was diminished on predictable vs. unpredictable trials for the CC group. The current findings suggest the ventromedial PFC plays a key role in modulating the emotional response to a controllable stressor. Further, these data provide a better understanding of the influence of predictability and controllability in the modulation of the threat-related response. 94 Introduction The ability to predict and control stressful events reduces the emotional response to future stressors (Amat et al., 2005; Baratta et al., 2007; Maier, 1986; Maier & Watkins, 2010; Weinberg et al., 2010). Moreover, exposure to unpredictable and uncontrollable stressful events is an important trigger in the development of anxiety-related disorders (Chorpita & Barlow, 1998). A stressor’s predictability is influenced by the environmental cues that signal it, whereas controllability is related to the ability to avoid, terminate, or moderate a stressor (Foa et al., 1992). In general, the emotional response to stress is diminished when stressors are predictable and controllable (Etkin, 2009; Maier et al., 2006), whereas exposure to unpredictable and uncontrollable stress is linked to anxietylike behaviors (Weinberg et al., 2010). The effect of predictability and controllability on brain-behavior relationships has primarily been investigated using animal models. This prior work has often employed a yoked Pavlovian fear conditioning paradigm. Typically in these studies, one group has the ability to terminate the unconditioned stimulus (UCS), whereas the yoked group cannot terminate the UCS (Maier et al., 2006). During Pavlovian conditioning a conditioned stimulus (CS) is paired with an aversive UCS. Associative learning is apparent when the CS generates a conditioned response (CR). The unconditioned response (UCR) elicited by the UCS is typically considered a reflexive, response that does not require associative learning. Prior animal research has shown that exposure to a controllable UCS interferes with subsequent fear conditioning, whereas exposure to an uncontrollable UCS enhances the conditioned fear-response (Baratta et al., 2007; Maier et al., 2006). The ventromedial prefrontal cortex (vmPFC) is a key brain region that 95 modulates the conditioned fear response to controllable stressors (Baratta et al., 2007; Maier et al., 2006). Prior human neuroimaging work also suggests that the prefrontal cortex (PFC) provides regulatory control over the amygdala during emotion-related tasks. Further, insufficient top-down regulatory control by the vmPFC results in an exaggerated amygdala response (Milad et al., 2006; Rauch et al., 2006). Although many prior conditioning studies have investigated brain activity in anticipation of a threat, relatively few studies have investigated the emotional response to the threat itself (Dunsmoor et al., 2008; Knight et al., 2010; Wood et al., 2012). However, the limited work that has been completed has demonstrated learning-related changes in UCR expression within the fMRI signal response of the dorsolateral PFC (dlPFC), dorsomedial PFC (dmPFC), vmPFC, and amygdala (Dunsmoor et al., 2008; Knight et al., 2010; Wood et al., 2012) that parallel the emotional response (indexed via SCR) (Knight et al., 2010; Wood et al., 2012). More specifically, UCR amplitude is diminished to predictable compared to unpredictable UCS presentations (Baxter, 1966; Dunsmoor et al., 2008; Knight et al., 2010, 2011; Lykken et al., 1972; Lykken & Tellegen, 1974; Peeke & Grings, 1968; Wood et al., 2012). This phenomenon is generally referred to as conditioned UCR diminution. Taken together this prior work suggests that predictability influences UCR expression. However, there has been limited research on the effect of controllability on UCR expression. The current study investigated the role of controllability and predictability in conditioned UCR diminution. To our knowledge, this is the first human neuroimaging study to employ a yoked procedure, similar to prior animal studies (Baratta et al., 2008; 96 Maier & Watkins, 2010), to investigate the effect of controllability and predictability on UCR expression. We hypothesized that controllability and predictability would diminish the magnitude of the unconditioned neurophysiological response. Materials and Methods Experimental Design: Participants were exposed to a differential fear conditioning procedure during fMRI in which the CS and UCS were presented through MRcompatible pneumatic headphones. The study consisted of yoked pairs of subjects, in which one group received a controllable UCS (Controllable Condition; CC) and the second group received an uncontrollable UCS (Uncontrollable Condition; UC). CC participants had the ability to terminate the UCS, whereas UC participants could not terminate the UCS. Participants: A total of fifty-four (27 CC and 27 UC) healthy right-handed volunteers participated in this study [28 female, 26 male; age = 23.39 ± 0.77 years (mean ± SEM); range = 18-38 years]. Participants in the two groups were matched on gender, ethnicity, age, and level of education (Table 1). There were no significant differences between the two groups based on these factors. All subjects provided written informed consent in compliance with the University of Alabama at Birmingham Institutional Review Board. State-Trait Anxiety Inventory: Participants completed the State-Trait Anxiety Inventory (STAI; Form Y) for Adults (Spielberger, 1983) after the conditioning session. The STAI consists of a self-assessment measure of state and trait anxiety in terms of general 97 negative affect (Grös et al., 2007). Scores on the state scale reflect anxiety level at the current moment, whereas trait anxiety scores reflect a relatively long-term predisposition for anxiety (Spielberger, 1983). Conditioned and unconditioned stimuli: Two tones (700 and 1300 Hz; 10 s duration; 20 s ITI) served as the CSs and a loud (100 dB) white-noise served as the UCS (duration: 0.56.0 s in 0.5 s increments). The UCS coterminated with one tone (CS+UCS) and the second tone was presented alone (CS−) during acquisition (two 960 s blocks). To assess conditioned diminution of the UCR, the acquisition phase also included presentations of the UCS alone. A total of 24 CS+UCS, 24 CS−, and 24 UCS alone trials were presented during acquisition (Figure 1). The stimuli were counterbalanced and presented in a pseudorandom order such that no more than two trials of the same stimulus were consecutively presented. UCS duration: CC participants were informed that the UCS would last between 0.5-6.0 s, and that they had the ability to control the duration of the UCS. They were informed that they could terminate the UCS by pressing a button on the joystick. In doing so, CC participants determined the duration of the UCS for themselves as well as their matched UC counterpart. UC participants were also informed that the UCS would last between 0.5-6.0 s. Given that UC participants did not have the ability to control the UCS, they were instructed to make a button press when the UCS ended, to control for motor activity associated with the button presses made by their match in the CC group. UCS expectancy: UCS expectancy was used as a measure of conscious expectation of the 98 UCS. Presentation software (Neurobehavioral Systems, Inc.; Albany, CA) was used to present a UCS expectancy rating scale on an IFIS-SA LCD (Invivo Corp.; Gainesville, FL) video screen located above the subject's head and viewed through a mirror attached to the RF coil. An MRI compatible joystick (Current Designs; Philadelphia, PA) was used to monitor subjects’ expectancy of receiving the UCS. The joystick controlled a rating bar which was presented throughout the conditioning session on the video screen. Subjects were instructed to rate their UCS expectancy from moment to moment using a continuous scale from 0 to 100 (0 = certain the UCS would not be presented, 50 = uncertain whether the UCS would be presented, 100 = certain the UCS would be presented) to reflect their current UCS expectancy. UCS expectancy was calculated as the average response (1 s sample) at UCS onset. Additional details on this methodology have been published previously (Knight & Wood, 2011). Skin conductance: An MRI compatible physiological monitoring system (Biopac Systems; Goleta, CA) was used to collect SCR data. SCR was sampled (10 kHz) with a pair of disposable radio-translucent dry electrodes (EL509, Biopac Systems; Goleta, CA). Isotonic recording electrode gel (Gel101, Biopac Systems; Goleta, CA) was applied to the electrodes which were then affixed to the thenar and hypothenar eminences of the left palm. SCR data were processed using Biopac AcqKnowledge 4.1 software. A 1 Hz low pass digital filter was applied and SCR data were resampled at 250 Hz. Unconditioned SCRs were limited to those that occurred within 10 s following the UCS presentation. Unconditioned SCRs smaller than 0.05 uSiemens were scored as 0. Data were then square root transformed prior to statistical analyses. 99 Electromyography: The MRI compatible physiological monitoring system (Biopac Systems; Goleta, CA) was also used to collect EMG data. EMG was sampled (10 kHz) with a pair of disposable radio-translucent electrodes (1 cm diameter, Biopac Systems; Goleta, CA) from the orbicularis oculi muscle below the left eye. The first electrode was placed directly below the left pupil while the second was placed laterally to the first electrode as per previous committee report guidelines (Blumenthal et al., 2005). EMG data were processed using Biopac AcqKnowledge 4.1 software. Following guidelines for digital filtering (Cook & Miller, 1992) and EMG denoising (Blumenthal et al., 2005) a Fast Fourier Transform was used to assess and remove frequency domains where noise occurred (Comb Band Stop filter at fMRI fundamental frequency ≈ 17.0 Hz, 60 Hz Notch filter, 28-400 Hz Kaiser-Bessel Band Pass filter). The EMG signal was resampled at 1000 Hz then rectified and integrated (20 ms time constant) for scoring. Responses were scored as the peak-valley difference with the valley occurring in the first 20 ms after the UCS and the peak occurring within the 21-150 ms window following the UCS (Blumenthal et al., 2005). Negative responses were scored as a zero. Functional MRI: Structural and functional imaging was completed on a 3 Tesla Siemens Allegra scanner. High-resolution anatomical images (MPRAGE) were obtained in the sagittal plane using a T1 weighted series (TR=2300 ms, TE=3.9 ms, flip angle=12⁰, FOV=25.6 cm, matrix=256 x 256, slice thickness=1 mm, 0.5 mm gap) to serve as an anatomical reference. Blood oxygen level dependent fMRI of the entire brain was conducted using a gradient-echo echoplanar pulse sequence in an oblique-axial orientation (TR=2000 ms, TE=30 ms, flip angle=70º, FOV=24 cm, matrix=64 x 64, slice 100 thickness=4 mm, no gap) during each block of stimulus presentations. Functional image processing was performed with the Analysis of Functional NeuroImages (AFNI) software package (Cox, 1996). Echo-planar time series data were corrected for slice timing offset, motion corrected, concatenated, reregistered to the fifth volume of the first imaging block, and spatially blurred using a 4 mm full-width-at-half-maximum Gaussian filter. Functional MRI data were analyzed at the individual subject level using the input from all stimuli in a multiple linear regression using a gamma variate hemodynamic response function. Regressors to account for brain activity not related to the UCR included reference waveforms for the CS+ and CS−, joystick movement, button presses, and head motion parameters. The regressors of interest for this study modeled the unconditioned fMRI signal response to UCS presentations during the CS+UCS and UCS alone. Percent signal change was used as an index of the magnitude of the unconditioned fMRI signal response produced by the UCS. Functional maps reflecting percent signal change were converted to the Talairach and Tournoux stereotaxic coordinate system for group analyses (Talairach & Tournoux, 1988). Based on prior work (Dunsmoor et al., 2008; Knight et al., 2010; Wood et al., 2012), an anatomical mask was used to restrict group level analyses to the PFC, cingulate cortex, IPL, insula, amygdala, and hippocampus to reduce the number of voxel-wise comparisons. We conducted a repeated-measures ANOVA to test for a main effect of predictability (CS+UCS vs. UCS alone) and controllability (CC vs. UC), as well as a predictability x controllability interaction. A voxel-wise threshold of p < 0.05 (corrected) was employed by using an uncorrected threshold of p < 0.005 and a cluster volume larger than 563 mm3 (10 voxels of 3.75 x 3.75 x 4.00 mm dimension). Given our a priori 101 hypotheses and relatively small volume of the amygdala and hippocampus we used a voxel-wise threshold of p < 0.005 and a cluster volume larger than 112 mm3 (2 voxels 3.75 x 3.75 x 4.00 mm dimension) to assess activity within these brain regions. These threshold criteria were used to correct for multiple comparisons based on Monte Carlo simulations that were used to reject smaller clusters of activation produced by chance alone (Forman et al., 1995; Saad et al., 2006) and result in family-wise error corrected significance of threshold p < 0.05. Follow-up t-test comparisons were conducted in SPSS on the mean percent signal change activation passing the significance threshold (p < 0.05 corrected) for the ANOVA. A secondary multiple linear regression analysis was completed to investigate the relationship between our behavioral measures (i.e. state anxiety, UCS expectancy, SCR, EMG) and the unconditioned fMRI signal response from brain regions that demonstrated learning-related changes in the ANOVA (i.e. functional regions of interest; ROI). The regression analysis evaluated these relationships on a voxel-wise basis. AlphaSim (Cox, 1996; Saad et al., 2006) was used to conduct Monte Carlo simulations limited to the functional ROI from our repeated-measures ANOVA that demonstrated a main effect of predictability, a main effect of controllability, or predictability x controllability interaction (p < 0.05; corrected). Prior work has demonstrated a relationship between the magnitude of the fMRI signal response within the amygdala, SCR, and EMG response during Pavlovian fear conditioning (Cheng et al., 2003, 2006, 2007; Knight et al., 2005, 2010; van Well et al., 2012; Wood et al., 2012). Additionally, prior human fear conditioning studies have demonstrated learning-related changes within the hippocampus (Knight et al., 2004, van Well et al., 2012). Therefore an anatomical mask was employed 102 to include the amygdala and hippocampus in the group level regression analysis. A voxelwise threshold of p < 0.005 and cluster volume larger than 112 mm3 (2 voxels 3.75 x 3.75 x 4.00 mm dimension) was employed, resulting in a family-wise error corrected significance threshold of p < 0.05. Results UCS duration: Given that CC and UC participants were yoked, there were no differences in UCS duration between the groups. In addition, there were no differences in UCS duration between the CS+UCS (mean = 3.51 ± 0.41; range = 0.5 – 6.0 s) and UCS alone (mean = 3.62 ± 0.40; range = 0.5 – 6.0 s; t[26] = -1.38, n.s.) trials. UCS expectancy: Repeated measures ANOVA revealed significant differences in UCS expectancy. Results showed a main effect for predictability (F[1,52] = 32.17, p < 0.05), but no main effect for controllability (F < 1.00) or predictability x controllability interaction (F < 1.00). UCS expectancy was greater on CS+UCS (mean ± SEM: 75.55 ± 2.93) than on UCS alone (60.47 ± 3.56) trials for the CC group (t[26] = 3.22, p < 0.05). The UC group also showed greater UCS expectancy on CS+UCS (76.95 ± 2.95) than UCS alone (57.92 ± 2.70; t[26] = 5.04, p < 0.05) trials (Figure 2a). The aim of this study was to investigate the effect of controllability and predictability on UCR expression. Therefore, contrasts including the CS− were not conducted because a UCS was not presented on CS− trials. 103 Skin conductance: Repeated measures ANOVA also revealed significant differences in unconditioned SCR expression. There was a main effect for predictability (F[1,52] = 15.71, p < 0.05), but no main effect for controllability (F = 1.75) or a predictability x controllability interaction (F < 1.00). T-test comparisons revealed a significantly diminished unconditioned SCR for CS+UCS trials (0.55 ± 0.09) compared to UCS alone trials (0.65 ± 0.12; t[26] = -2.23, p < 0.05) for CC participants. The same pattern was observed for UC participants. Unconditioned SCRs were diminished on CS+UCS trials (0.71 ± 0.09) compared to UCS alone trials (0.85 ± 0.09; t[26] = -3.44; p < 0.05) (Figure 2b). Electromyography: Repeated measures ANOVA also revealed significant differences in the startle-eyeblink response. There was a main effect for predictability (F[1,52] = 11.28, p < 0.05), but no main effect for controllability (F = 1.11) or predictability x controllability interaction (F = 2.80). T-test comparisons revealed a significantly enhanced startle-eyeblink response on CS+UCS trials (289.65 ± 39.39) compared to UCS alone trials (191.32 ± 27.69; t[26] = 2.97, p < 0.05) for CC participants. There was no difference in EMG response for UC participants on CS+UCS trials (218.87 ± 23.55) compared to UCS alone trials (185.92 ± 22.48; t[26] = 1.57, n.s.) (Figure 2c). Functional MRI: Repeated measures ANOVA revealed significant differences in the magnitude of the unconditioned fMRI signal response within several brain regions (Table 2, Figures 3-4). In each of these regions the unconditioned fMRI signal response demonstrated a main effect for predictability (F[53] > 8.61; p < 0.05 corrected). There 104 was not a main effect for controllability. A predictability x controllability interaction was observed within vmPFC and left hippocampus (Table 2; Figure 3). T-test comparisons were completed on the mean fMRI signal from each volume of activation that passed the significance threshold (p < 0.05 corrected) for the main effect of stimulus type. All regions showed a diminished UCR on CS+UCS vs. UCS alone trials. Post hoc contrasts on the predictability x controllability interaction were conducted on the mean fMRI signal from the vmPFC and left hippocampus. These t-test comparisons revealed a diminished UCR on CS+UCS vs. UCS alone trials for the CC group within the vmPFC (t[26] = -3.93; p < 0.05 corrected) and left hippocampus (t[26] = -4.38; p < 0.05 corrected). The fMRI signal response on CS+UCS vs. UCS alone trials for the UC group was not significantly different once corrected for multiple comparisons (vmPFC t[26] = 2.03; p = n.s.; left hippocampus t[26] = 2.70, p = n.s.). There were no group differences revealed in the post hoc contrasts. Specifically, no differences were observed between the CC group compared to the UC group on CS+UCS or UCS alone trials. The fMRI signal response on CS+UCS trials for the CC group vs. UC group was not significantly different once corrected for multiple comparisons (vmPFC t[26] = -2.35; p = n.s.; left hippocampus t[26] = -2.32; p = n.s.). Similarly, there was no difference in the fMRI signal response on UCS alone trials for the CC group vs. UC group (vmPFC t[26] = 1.66, p = n.s.; left hippocampus t[26] = 1.24, p = n.s.). A voxel-wise multiple linear regression analysis was conducted separately on the averaged [predictable (i.e. CS+UCS) and unpredictable (i.e. UCS alone)] threat-related fMRI signal response, as well as the difference (predictable – unpredictable) in activation between these trial types. The linear regression model accounted for state anxiety, UCS 105 expectancy, SCR production, and EMG response. These analyses were restricted to the functional ROI identified from the ANOVA with one exception. The subcortical structures of interest (i.e. amygdala and hippocampus) were also included based on prior human fear conditioning studies that have demonstrated learning-related changes within these brain areas (Bach et al., 2011; Knight et al., 2005, 2004; van Well et al., 2012). The regression analysis on the averaged threat-related fMRI signal response demonstrated that state anxiety level explained unique variability in the activation observed within dmPFC, vmPFC, and posterior cingulate (PCC) (Table 3). There were no brain regions that showed a relationship with UCS expectancy, SCR, or EMG that met our significance criteria. No other significant differences were observed. Discussion The predictability and controllability of stressors impacts our emotional response to stressful events. Typically, the emotional response to a stressor is diminished when events are predictable and controllable (Etkin, 2009; Maier et al., 2006). However, exposure to unpredictable and uncontrollable stress results in an enhanced stress response and is linked to anxiety-like behaviors (Weinberg et al., 2010). The present study employed a unique Pavlovian fear conditioning procedure to investigate the effect of predictability and controllability on the emotional response to a threat. During Pavlovian conditioning, participants are typically exposed to an uncontrollable UCS. However, this study consisted of two groups, a CC group that could terminate the UCS and a traditional UC group that could not terminate the UCS. Another novel quality of the current study was that participants were yoked, similar to paradigms used in animal research (Baratta et 106 al., 2008; Maier & Watkins, 2010). Additionally, the current procedure included predictable and unpredictable presentations of the UCS to investigate conditioned UCR diminution. We also assessed state anxiety, UCS expectancy, unconditioned SCR, startle eye-blink EMG, and the fMRI signal response during Pavlovian conditioned UCR diminution to better understand the processes that modulate the threat-related response. In the current study, learning-related changes were observed in our behavioral data. Both CC and UC groups demonstrated high UCS expectancy ratings for predictable compared to unpredictable trials (Figure 2a). These findings demonstrate that participants learned that the CS+ predicted the UCS and remained uncertain of the timing of UCS alone presentations. As anticipated, there were no group differences in UCS expectancy. Similar to prior work, conditioned diminution of the unconditioned SCR was also observed in the present study. The amplitude of unconditioned SCR was diminished on predictable compared to unpredictable trials for both the CC and UC groups. These findings are consistent with prior behavioral studies that have demonstrated a reduction in UCR magnitude to predictable compared to unpredictable UCS presentations (Baxter, 1966; Kimmel, 1967; Knight et al., 2011; Lykken et al., 1972; Marcos & Redondo, 1999). Learning-related changes within the EMG response were also observed in the current study. Specifically, the EMG response was enhanced for the CC group on predictable compared to unpredictable trials. This finding is similar to prior work that demonstrated an enhanced startle response during a CS compared to baseline startle (Grillon & Davis, 1997). There were no differences in EMG response for predictable vs. unpredictable trials for the UC group. Additionally, no group differences were observed in threat-related SCR expression or EMG response. Group differences in threat-elicited 107 SCR and EMG may have been masked by the degree of between-subject variability often observed within these measures. Taken together, these findings are similar to previous human fear conditioning studies and suggest that predictability influences UCR expression (Baxter, 1966; Kimmel, 1967; Knight et al., 2011; Lykken et al., 1972; Marcos & Redondo, 1999; Wood et al., 2012). Similar to prior work, the threat-related fMRI signal response paralleled unconditioned SCR expression. Specifically, a diminished fMRI signal response within dlPFC, dmPFC, vmPFC, vlPFC, and PCC was observed to predictable compared to unpredictable presentations of the UCS. These findings replicate prior research designed to investigate learning-related changes within the threat-related fMRI signal response (Wood et al., 2012). This pattern was not observed between the EMG data and the threatrelated fMRI signal response. Although there were no group differences in the startleeyeblink response, the CC group did demonstrate an enhanced startle-eyeblink response to predictable (i.e. CS+UCS) compared to unpredictable (i.e. UCS alone) trials. The UC group did not show learning-related changes in the EMG response. Specifically, no difference in startle-eyeblink response was observed for predictable vs. unpredictable UCS presentations for the UC group. Somewhat consistent with this weak behavioral effect, controllability influenced the threat-related fMRI signal response within the vmPFC and left hippocampus, such that the response within these brain regions was diminished when the threat was both predictable and controllable. However, post hoc analyses of the predictability x controllability interaction within the vmPFC and left hippocampus did not reveal significant group differences. Specifically, there was no difference in the amplitude of the fMRI signal response for the CC group compared to the 108 UC group on predictable or unpredictable trials. Since there was not a predictability x controllability interaction observed in our behavioral data (i.e. UCS expectancy, SCR, or EMG), the predictability x controllability interaction observed within the vmPFC and left hippocampus is difficult to interpret. Given our focus on brain-behavior relationships, the interaction effect observed in the imaging data would be more compelling if a similar pattern was observed in the behavioral data. Prior animal studies have shown that the vmPFC plays an important role in modulating conditioned fear responses to controllable stressors (Baratta et al., 2007, 2008; Maier et al., 2006). Additionally, recent human neuroimaging research has demonstrated controllability enhances anticipatory fear activity within the vmPFC (Kerr et al., 2012). However, to our knowledge this is the first study to demonstrate that controllability affects the vmPFC response to the threat itself. Further, prior work has shown that the vmPFC is an important region that provides regulatory control over the amygdala during emotion-related tasks (Milad et al., 2006; Rauch et al., 2006). Perhaps, the exaggerated EMG response among the CC group is partly due to the diminished threat-related fMRI signal response observed within the vmPFC. For example, prior work suggests that regions of the PFC modulate subcortical brain areas (e.g. the amygdala) that control the peripheral expression of emotion (Etkin & Wager, 2007; Kim & Jung, 2006; Milad et al., 2004; Phillips et al., 2003). Therefore, diminished threatrelated activation within the vmPFC may contribute to the enhanced EMG response observed on predictable trials for the CC group. Prior work also suggests that anxiety level influences top-down mechanisms that support regulatory control processes (Basten et al., 2011; Delgado et al., 2008; Klumpp et 109 al., 2011; Nitschke et al., 2006; Ochsner et al., 2002; Sehlmeyer et al., 2011). For example, prior work suggests that anxiety level influences the magnitude of anticipatory brain activation within the vmPFC (Indovina et al., 2011). More specifically, high anxiety is associated with diminished vmPFC activity and an enhanced emotional response (indexed via SCR) (Indovina et al., 2011). We have previously demonstrated anxiety level varied with the threat-related fMRI signal response within the dlPFC, dmPFC, PCC, and IPL (Wood et al., 2012). In this prior work, as anxiety level increased the threatrelated fMRI signal response within these brain regions also increased (Wood et al., 2012). A positive relationship was observed between state anxiety and the threat-related fMRI signal response within dmPFC, vmPFC, and PCC in the current study. Taken together, these findings suggest that healthy emotion regulation relies upon the PFC (Basten et al., 2011; Delgado et al., 2008; Nitschke et al., 2006; Ochsner et al., 2002). In summary, learning-related changes were observed within the threat-related neurophysiological response during Pavlovian fear conditioning. Conditioned UCR diminution was observed within dlPFC, dmPFC, vmPFC, vlPFC, and PCC. Threatelicited SCR expression paralleled activation within the brain regions that showed UCR diminution. However, the opposite pattern was observed for the EMG response in relation to brain activity. Similar to our prior work, the predictability of a threat affected the threat-related neurophysiological response that was elicited. Further, the controllability of a threat also influenced the fMRI signal response within the vmPFC and left hippocampus. Specifically, the magnitude of the threat-related response within these brain regions was diminished when the threat was both controllable and predictable. This finding suggests that the controllability and predictability of a threat impacts the 110 magnitude of both cortical and subcortical brain activation in response to a threat. To our knowledge, this is the first human study to demonstrate the impact of predictability and controllability on the threat-elicited neural response. However, a behavioral component showing a similar pattern is lacking in the current results. Our inability to detect a significant effect may be due to the degree of between-subject variability in the psychophysiological data. Future studies, should examine the influence of predictability and controllability on the threat-elicited emotional response using a within-subjects design. Finally, the data suggest that the vmPFC and hippocampus play a key role in modulating the fear response to a controllable stressor (Baratta et al., 2008; Kerr et al., 2012; Maier et al., 2006). Taken together, the findings from the current study provide evidence that both predictability and controllability of a stressor influences the neurophysiological response to a threat. Acknowledgements: This research was supported by the University of Alabama at Birmingham Faculty Development Grant Program and NIH R01 MH098348 (DCK). 111 References Amat, J., Baratta, M., Paul, E., Bland, S., Watkins, L., & Maier, S. (2005). Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nature neuroscience, 8(3), 365–71. doi:10.1038/nn1399 Bach, D. R., Weiskopf, N., & Dolan, R. J. (2011). A stable sparse fear memory trace in human amygdala. The Journal of neuroscience: the official journal of the Society for Neuroscience, 31(25), 9383–9. doi:10.1523/JNEUROSCI.1524-11.2011 Baratta, M. V, Christianson, J. P., Gomez, D. M., Zarza, C. M., Amat, J., Masini, C. V, Watkins, L. R., et al. (2007). Controllable versus uncontrollable stressors bidirectionally modulate conditioned but not innate fear. Neuroscience, 146(4), 1495– 503. doi:10.1016/j.neuroscience.2007.03.042 Baratta, M. V, Lucero, T. R., Amat, J., Watkins, L. R., & Maier, S. F. (2008). Role of the ventral medial prefrontal cortex in mediating behavioral control-induced reduction of later conditioned fear. Learning & memory (Cold Spring Harbor, N.Y.), 15(2), 84–7. doi:10.1101/lm.800308 Basten, U., Stelzel, C., & Fiebach, C. J. (2011). Trait Anxiety Modulates the Neural Efficiency of Inhibitory Control. Journal of cognitive neuroscience, (10), 3132– 3145. Baxter, R. (1966). Diminution and recovery of the UCR in delayed and trace classical GSR conditioning. Journal of experimental psychology, 71(3), 447–451. Blumenthal, T. D., Cuthbert, B. N., Filion, D. L., Hackley, S., Lipp, O. V, & Van Boxtel, A. (2005). Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology, 42(1), 1–15. doi:10.1111/j.14698986.2005.00271.x Cheng, D. T., Knight, D. C., Smith, C. N., & Helmstetter, F. J. (2006). Human amygdala activity during the expression of fear responses. Behavioral neuroscience, 120(6), 1187–95. doi:10.1037/0735-7044.120.5.1187 Cheng, D. T., Knight, D. C., Smith, C. N., Stein, E. A., & Helmstetter, F. J. (2003). Functional MRI of human amygdala activity during Pavlovian fear conditioning: Stimulus processing versus response expression. Behavioral Neuroscience, 117(1), 3–10. doi:10.1037/0735-7044.117.1.3 Cheng, D. T., Richards, J., & Helmstetter, F. J. (2007). Activity in the human amygdala corresponds to early, rather than late period autonomic responses to a signal for shock. Learning & Memory, 14(7), 485–490. doi:10.1101/lm.632007.sion 112 Chorpita, B. F., & Barlow, D. H. (1998). The development of anxiety: the role of control in the early environment. Psychological bulletin, 124(1), 3–21. Cook, E. W. 3rd, & Miller, A. M. (1992). Methodology Digital Filtering: Background and Tutorial for Psychophysiologists. Psychophysiology, 29(3), 350–367. Cox, R. W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and biomedical research, an international journal, 29(3), 162–73. Delgado, M. R., Nearing, K. I., Ledoux, J. E., & Phelps, E. A. (2008). Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron, 59(5), 829–38. doi:10.1016/j.neuron.2008.06.029 Dunsmoor, J. E., Bandettini, P. A., & Knight, D. C. (2008). Neural correlates of unconditioned response diminution during Pavlovian conditioning. NeuroImage, 40(2), 811–7. doi:10.1016/j.neuroimage.2007.11.042 Etkin, A. (2009). Functional Neuroanatomy of Anxiety: A Neural Circuit Perspective. Behavioral neurobiology of Anxiety and its treatment, Current topics in Behavioral Neurosciences 2 (pp. 251–277). doi:10.1007/7854 Etkin, A., & Wager, T. D. (2007). Functional Neuroimaging of Anxiety: A MetaAnalysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. American Journal of Psychiatry, 164(October), 1476–1488. Foa, E. B., Zinbarg, R., & Rothbaum, B. O. (1992). Uncontrollability and unpredictability in post-traumatic stress disorder: an animal model. Psychological bulletin, 112(2), 218–38. Forman, S., Cohen, J., Fitzgerald, M., Eddy, W., Mintun, M., & Noll, D. (1995). Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic resonance in medicine, 33(5), 636–647. Grillon, C., & Davis, M. (1997). Fear-potentiated startle conditioning in humans: Explicit and contextual cue conditioning following paired versus unpaired training. Psychophysiology, (34), 451–458. Grös, D. F., Antony, M. M., Simms, L. J., & McCabe, R. E. (2007). Psychometric properties of the State-Trait Inventory for Cognitive and Somatic Anxiety (STICSA): Comparison to the State-Trait Anxiety Inventory (STAI). Psychological assessment, 19(4), 369–81. doi:10.1037/1040-3590.19.4.369 113 Indovina, I., Robbins, T. W., Núñez-Elizalde, A. O., Dunn, B. D., & Bishop, S. J. (2011). Fear-conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron, 69(3), 563–71. doi:10.1016/j.neuron.2010.12.034 Kerr, D. L., McLaren, D. G., Mathy, R. M., & Nitschke, J. B. (2012). Controllability Modulates the Anticipatory Response in the Human Ventromedial Prefrontal Cortex. Frontiers in Psychology, 3(December), 1–11. doi:10.3389/fpsyg.2012.00557 Kim, J. J., & Jung, M. W. (2006). Neural circuits and mechanisms involved in Pavlovian fear conditioning: A critical review. Neuroscience & Biobehavioral Reviews, 30(2), 188–202. Kimmel, E. (1967). Judgments of UCS intensity and diminution of the UCR in classical GSR conditioning. Journal of experimental psychology, 73, 532–543. Klumpp, H., Ho, S. S., Taylor, S. F., Phan, K. L., Abelson, J. L., & Liberzon, I. (2011). Trait anxiety modulates anterior cingulate activation to threat interference. Depression and anxiety, 28(3), 194–201. doi:10.1002/da.20802 Knight, D. C., Lewis, E. P., & Wood, K. H. (2011). Conditioned diminution of the unconditioned skin conductance response. Behavioral neuroscience, 125(4), 626– 31. doi:10.1037/a0024324 Knight, D. C., Nguyen, H. T., & Bandettini, P. A. (2005). The role of the human amygdala in the production of conditioned fear responses. NeuroImage, 26(4), 1193–200. doi:10.1016/j.neuroimage.2005.03.020 Knight, D. C., Smith, C. N., Cheng, D. T., Stein, E. A., & Helmstetter, F. J. (2004). Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cognitive, affective & behavioral neuroscience, 4(3), 317–25. Knight, D. C., Waters, N. S., King, M. K., & Bandettini, P. A. (2010). Learning-related diminution of unconditioned SCR and fMRI signal responses. NeuroImage, 49(1), 843–8. doi:10.1016/j.neuroimage.2009.07.012 Knight, D. C., & Wood, K. H. (2011). Investigating the neural mechanisms of aware and unaware fear memory with FMRI. Journal of visualized experiments: JoVE, (56), 1– 6. doi:10.3791/3083 Lykken, D. T., Macindoe, I., & Tellegen, A. (1972). Preception: Autonomic Response to Shock as a Function of Predictability in Time and Locus. Psychophysiology, 9(3), 318–333. Lykken, D. T., & Tellegen, A. (1974). On the validity of the preception hypothesis. Psychophysiology, 11(2), 125–32. 114 Maier, S. F. (1986). Stressor controllability and stress-induced analgesia. Annals of the New York Academy of Sciences, 467, 55–72. Maier, S. F., Amat, J., Baratta, M. V, Paul, E., & Watkins, L. R. (2006). Behavioral control, the medial prefrontal cortex, and resilience. Dialogues in Clinical Neuroscience, 8(4), 397–407. Maier, S. F., & Watkins, L. R. (2010). Role of the medial prefrontal cortex in coping and resilience. Brain research, 1355, 52–60. doi:10.1016/j.brainres.2010.08.039. Marcos, J. L., & Redondo, J. (1999). Effects of conditioned stimulus presentation on diminution of the unconditioned response in aversive classical conditioning. Biological psychology, 50(2), 89–102. Milad, M. R., Rauch, S. L., Pitman, R. K., & Quirk, G. J. (2006). Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biological psychology, 73(1), 61–71. doi:10.1016/j.biopsycho.2006.01.008 Milad, M. R., Vidal-Gonzalez, I., & Quirk, G. J. (2004). Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behavioral neuroscience, 118(2), 389–94. doi:10.1037/0735-7044.118.2.389 Nitschke, J. B., Sarinopoulos, I., Mackiewicz, K. L., Schaefer, H. S., & Davidson, R. J. (2006). Functional neuroanatomy of aversion and its anticipation. NeuroImage, 29(1), 106–16. doi:10.1016/j.neuroimage.2005.06.068 Ochsner, K. N., Bunge, S. A., Gross, J. J., & Gabrieli, J. D. (2002). Rethinking feelings: an fMRI study of the cognitive regulation of emotion. Journal of cognitive neuroscience, 14, 1215–1229. Peeke, S. C., & Grings, W. W. (1968). Magnitude of UCR as a function of variability in the CS–UCS relationship. Journal of experimental psychology, 77, 1–6. Phillips, M. L., Drevets, W. C., Rauch, S. L., & Lane, R. (2003). Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biological Psychiatry, 54(5), 504–514. doi:10.1016/S0006-3223(03)00168-9 Rauch, S. L., Shin, L. M., & Phelps, E. A. (2006). Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biological psychiatry, 60(4), 376–82. doi:10.1016/j.biopsych.2006.06.004 Saad, Z. S., Chen, G., Reynolds, R. C., Christidis, P. P., Hammett, K. R., Bellgowan, P. S. F., & Cox, R. W. (2006). Functional imaging analysis contest (FIAC) analysis according to AFNI and SUMA. Human brain mapping, 27(5), 417–24. doi:10.1002/hbm.20247 115 Sehlmeyer, C., Dannlowski, U., Schöning, S., Kugel, H., Pyka, M., Pfleiderer, B., Zwitserlood, P., et al. (2011). Neural correlates of trait anxiety in fear extinction. Psychological medicine, 41(4), 789–98. doi:10.1017/S0033291710001248 Spielberger, C. (1983). State–Trait Anxiety Inventory for Adults. Redwood City, CA: Mind Garden. Talairach, J., & Tournoux, P. (1988). Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme. Van Well, S., Visser, R. M., Scholte, H. S., & Kindt, M. (2012). Neural substrates of individual differences in human fear learning: Evidence from concurrent fMRI, fearpotentiated startle, and US-expectancy data. Cognitive, affective & behavioral neuroscience. doi:10.3758/s13415-012-0089-7 Weinberg, M. S., Grissom, N., Paul, E., Bhatnagar, S., Maier, S. F., & Spencer, R. L. (2010). Inescapable but not escapable stress leads to increased struggling behavior and basolateral amygdala c-fos gene expression in response to subsequent novel stress challenge. Neuroscience, 170(1), 138–148. doi:10.1016/j.neuroscience.2010.06.052. Wood, K. H., Ver Hoef, L. W., & Knight, D. C. (2012). Neural mechanisms underlying the conditioned diminution of the unconditioned fear response. NeuroImage, 60(1), 787–99. doi:10.1016/j.neuroimage.2011.12.048 116 Table 1. Demographics and group characteristics. Controllable Uncontrollable Measures Condition Condition Male/Female 13/14 13/14 Age 23.37 ± 1.10 23.41 ± 1.11 range 18-38 18-38 Education (years) 14.89 ± 0.49 15.26 ± 0.59 range 12-22 12-21 State Anxiety 33.30 ± 1.47 35.30 ± 1.49 Trait Anxiety 36.93 ± 1.71 38.63 ± 1.23 117 t p −0.03 n.s. −0.68 n.s. −1.05 −0.81 n.s. n.s. Table 2. Regions showing conditioned diminution of the UCR. Region Vol (mm3) Main Effect of Predictability Dorsolateral PFC Right 747 Left 4034 Left 834 Dorsomedial PFC 13196 Talairach coordinates x y z CS+UCS vs. UCS alone t 31.9 −32.3 −26.6 3.1 0.2 0.0 45.7 13.2 57.3 53.1 31.8 40.1 −4.03 −4.58 −4.29 −4.75 Ventromedial PFC Right Left 2245 975 7.6 −3.2 38.8 36.5 7.0 6.8 −4.45 −4.12 Ventrolateral PFC Left 705 −28.2 16.2 −15.8 −4.33 Posterior Cingulate 5902 2.0 −33.3 29.8 −4.67 Predictability x Controllability Interaction Ventromedial PFC 837 2.0 39.6 −12.7 CC Group −3.93 UC Group n.s. Hippocampus 586 −33.1 −23.4 −11.5 CC Group −4.39 UC Group n.s. Note. Location, volumes, and coordinates from Talairach and Tournoux (1988) for the center of mass for areas of activation. Significance criteria: ANOVA F[53] > 8.61, p < 0.05 (corrected); t[53] p < 0.05 (corrected). 118 Table 3. Regional activity varying with state anxiety. Talairach coordinates Region Hemisphere Vol (mm3) x y z Dorsomedial PFC Left 306 −5.3 29.7 36.9 Ventromedial PFC Bilateral 1211 5.0 38.8 7.1 Posterior Cingulate Bilateral 2256 −0.6 −33.9 27.9 Note. Location, volumes, and coordinates from Talairach and Tournoux (1988) for the center of mass for areas of activation. 119 Acquisition Phase CS+ UCS CSUCS No CS UCS 0 10 20 30 40 Time (Seconds) 50 Figure 1. Acquisition Phase. During the acquisition phase, participants received a total of 24 presentations of each stimulus (24 CS+UCS, 24 CS−, and 24 UCS alone). The CS+ coterminated with the UCS, the CS− was presented alone, and UCS alone trials were not preceded by a CS. 120 UCS Expectancy 100 a CC UC 80 60 40 20 µSiemens 1.0 b 0.8 0.6 0.4 0.2 350 c µVolts 300 250 200 150 Predictable Unpredictable Figure 2. UCS expectancy, unconditioned SCR, and EMG response. a) Both the CC and UC groups demonstrated learning-related differences in UCS expectancy. UCS expectancy was higher to the predictable (i.e. CS+UCS) compared to unpredictable (i.e. UCS alone) trials. b) Learning related changes in unconditioned SCR expression were also observed for both the CC and UC groups. Unconditioned SCRs were diminished on predictable vs. unpredictable trials. c) The CC group demonstrated learning-related changes within the EMG response. The EMG response was enhanced on predictable trials compared to unpredictable trials for the CC group. There were no differences in EMG response for the UC group. No group differences were observed in UCS expectancy, unconditioned SCR, or EMG. 121 x=6 0.3 0.2 Ventromedial PFC 0.1 Predictable Unpredictable 0.20 * fMRI signal (% ) 0.25 fMRI signal (% ) fMRI signal (% ) 0.4 Dorsomedial PFC * Posterior Cingulate * 0.20 0.15 0.10 Predictable 0.15 Unpredictable 0.10 0.05 Predictable Unpredictable Figure 3. Conditioned UCR diminution within the fMRI signal response. Significant diminution of the unconditioned fMRI signal response was observed within several brain regions (see Table 2) including prefrontal cortex (PFC) and Posterior Cingulate (PCC). No differences were observed between the CC and UC groups. UCR amplitude within each of these brain areas was reduced on predictable (i.e. CS+UCS) compared to unpredictable (i.e. UCS alone) trials. Graphs reflect the mean amplitude (% signal change) of all voxels within volumes of activation. Asterisk indicates significant difference. 122 Ventromedial PFC fMRI signal (% ) 0.10 0.05 0.00 -0.05 -0.10 Predictable Unpredictable x=3 Hippocampus fMRI signal (% ) 0.10 Right 0.05 0.00 -0.05 CC UC -0.10 Left Predictable y = -21 Unpredictable Figure 4. Regions showing predictability x controllability interaction. The unconditioned fMRI signal response within ventromedial PFC and left hippocampus was diminished on predictable trials compared to unpredictable trials for the CC group, but not the UC group. Graphs reflect the mean amplitude (% signal change) of all voxels within the volume of activation. 123 SUMMARY Typically, the response to a warning cue in anticipation of a threat is the primary focus of Pavlovian conditioning studies. Although this anticipatory response is often used as an index of associative learning, there are also associative learning-related changes in the unconditioned response (UCR) produced by the threat itself. From a functional perspective, it is important to understand learning-related changes in these innate UCRs to naturally occurring threats due to their biological relevance for survival (Domjan, 2005). This project employed Pavlovian fear conditioning to investigate the influence of associative learning, expectation, predictability, and controllability in the modulation of the neurophysiological response to a threat. In this project, we observed conditioned UCR diminution, using fMRI in conjunction with behavioral measures (e.g. SCR expression and UCS expectancy ratings) during the conditioning procedure, such that responses were diminished on predictable compared to unpredictable presentations of the UCS. More specifically, we observed conditioned diminution of the UCR within many of the same brain regions (e.g. dlPFC, dmPFC, and vmPFC) in each of the studies. Further, UCS expectancy varied with activity within several brain areas that showed UCR diminution. These findings demonstrate that our emotional response is minimized to aversive events that are predictable. Further, our studies have identified the neural circuitry that likely mediates this effect. Specifically, the PFC regulates amygdala activity and the amygdala produces the peripheral emotional response via projections to brainstem structures. These findings demonstrate that the PFC-amygdala circuit mediates the emotional response to a threat. 124 We also investigated UCS controllability, in addition to predictability, to better understand the neurophysiological mechanisms that affect conditioned UCR diminution. In general, we observed conditioned UCR diminution in the final study within many of the same brain areas (e.g. dlPFC, dmPFC, and vmPFC) as our initial investigations of predictability. A novel finding in the final study was the affect of controllability on the threat-related fMRI signal response within the vmPFC and hippocampus. Specifically, a diminished response was only observed when the UCS was both predictable and controllable. Given that predictability and controllability moderate the emotional response to stress, the current findings identify specific brain regions that may mediate the resilience to stress associated with predictable and controllable aversive events. Studying the biological mechanisms that contribute to learning-related changes in the fear response provides a starting point to better understand emotion dysregulation, such as that observed in anxiety disorders (Davis et al., 2009; Grillon, 2002; Kim & Jung, 2006; Milad et al., 2006). Prior investigations of the neurobiological markers of anxiety suggest that insufficient top-down regulatory control (Kim et al., 2011; Klumpp et al., 2011; Nitschke et al., 2006; Rauch et al., 2006; Schienle et al., 2010) results in hypersensitivity of subcortical brain areas (e.g. the amygdala) (Etkin & Wager, 2007; Milad et al., 2007, 2006). The current project extends this prior work by demonstrating that the predictability and controllability of a threat affects brain activity within the neural circuitry that regulates and expresses emotion. Further, this project demonstrates that individual differences in anxiety level influence the threat-elicited activity within these brain regions. 125 There are some limitations to this project, specifically in Aims #2 and #3. For example, prior work has demonstrated learning independent of expectation, in anticipation of a threat (Knight et al., 2003, 2006; Schultz & Helmstetter, 2010). The goal in Aim #2 was to assess UCR diminution independent of conscious expectations of the threat. However, differences in the threat response were not observed independent of expectation. Therefore, we were not able to address Aim #2 in this project. In Aim #3 we expected to observe an increased threat-elicited response for participants in the UC group compared to the CC group. However, no group differences were observed in the data. One possibility for this result is the large between-subject variability that is typically observed in these (i.e. SCR and startle EMG) psychophysiological measures. Additionally, the effect of controllability has primarily been observed in anticipation of a threat. However, Aim #3 investigated the influence of controllability on the threat-related response. The impact of controllability on the threat response may be more difficult to demonstrate compared to the influence of controllability in anticipation of a threat. Future studies may better elucidate the affect of controllability on threat-elicited activation by using a within-subject paradigm. Finally, learning-related changes in the neurophysiological response to a threat were observed in a healthy population. A future study of interest is to examine learning-related changes in the threat response using a patient population (e.g. post-traumatic stress disorder or major depressive disorder). Given the aberrant emotional regulation that is often found in anxiety disorders, we would expect to observe diminished activation within the PFC and increased activation of the amygdala in these groups compared to healthy individuals. 126 The work completed for this project provides a better understanding of the neurophysiological mechanisms that influence the response to a threat. Specifically, we have demonstrated learning-related changes in the neurophysiological response elicited by a threat. The primary focus of fear conditioning studies has typically been centered around the anticipatory response to a threat. This prior work has contributed to the development of cognitive, behavioral, and drug interventions (Jovanovic & Ressler, 2010; Milad et al., 2009; Ressler et al., 2004; Vervliet et al., 2004). However, the current studies have focused on the response to the threat itself. By demonstrating the influence of predictability and controllability in modulating the emotional response to a threat we have expanded the potential for novel treatments. For example, the findings from this project have revealed learning-related changes in the threat response of specific brain areas. Further, the psychophysiological response to a threat also showed learning-related changes. Future investigation of the threat-related response to predictable and/or controllable threats may provide additional insight for new cognitive, behavioral, or drug interventions. In summary, this project has contributed to a better understanding of the neural circuitry that supports fear-related processes. The findings from these studies demonstrate learning-related changes within the PFC-amygdala network that control the emotional response to a threat. Further, this work demonstrates that regions of the PFC regulate the emotional response controlled by the amygdala. Overall, the current findings provide a better understanding of the neural mechanisms that mediate the emotional response to a threat. 127 GENERAL LIST OF REFERENCES Amat, J., Baratta, M., Paul, E., Bland, S., Watkins, L., & Maier, S. (2005). Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nature neuroscience, 8(3), 365–71. doi:10.1038/nn1399 Baratta, M. V, Christianson, J. P., Gomez, D. M., Zarza, C. M., Amat, J., Masini, C. V, Watkins, L. R., et al. (2007). Controllable versus uncontrollable stressors bidirectionally modulate conditioned but not innate fear. Neuroscience, 146(4), 1495– 503. doi:10.1016/j.neuroscience.2007.03.042 Baratta, M. V, Lucero, T. R., Amat, J., Watkins, L. R., & Maier, S. F. (2008). Role of the ventral medial prefrontal cortex in mediating behavioral control-induced reduction of later conditioned fear. Learning & memory (Cold Spring Harbor, N.Y.), 15(2), 84–7. doi:10.1101/lm.800308 Baxter, R. (1966). Diminution and recovery of the UCR in delayed and trace classical GSR conditioning. Journal of experimental psychology, 71(3), 447–451. Bellgowan, P. S., & Helmstetter, F. J. (1996). Neural systems for the expression of hypoalgesia during nonassociative fear. Behavioral neuroscience, 110(4), 727–36. Büchel, C., Morris, J., Dolan, R. J., & Friston, K. J. (1998). Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron, 20(5), 947–57. Critchley, H. D., Mathias, C. J., & Dolan, R. J. (2001). Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron, 29(2), 537–45. Davis, M. (1992). The role of the amygdala in fear and anxiety. Annual review of neuroscience, 15, 353–75. doi:10.1146/annurev.ne.15.030192.002033 Davis, M., & Whalen, P. J. (2001). The amygdala: vigilance and emotion. Molecular psychiatry, 6(1), 13–34. Davis, M., Walker, D. L., Miles, L., & Grillon, C. (2009). Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology, 35(1), 1–31. doi:10.1038/npp.2009.109 Delgado, M. R., Nearing, K. I., Ledoux, J. E., & Phelps, E. A. (2008). Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron, 59(5), 829–38. doi:10.1016/j.neuron.2008.06.029 128 Domjan, M. (2005). Pavlovian conditioning: a functional perspective. Annual review of psychology, 56, 179–206. doi:10.1146/annurev.psych.55.090902.141409 Dunsmoor, J. E., Bandettini, P. A., & Knight, D. C. (2007). Impact of continuous versus intermittent CS-UCS pairing on human brain activation during Pavlovian fear conditioning. Behavioral neuroscience, 121(4), 635–42. doi:10.1037/07357044.121.4.635 Etkin, A., & Wager, T. D. (2007). Functional Neuroimaging of Anxiety: A MetaAnalysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. American Journal of Psychiatry, 164(October), 1476–1488. Fanselow, M. S., & Ledoux, J. E. (1999). Pavlovian fear conditioning occurs in the basolateral amygdala, 23, 229–232. Foa, E. B., Zinbarg, R., & Rothbaum, B. O. (1992). Uncontrollability and unpredictability in post-traumatic stress disorder: an animal model. Psychological bulletin, 112(2), 218–38. Grillon, C. (2002). Startle reactivity and anxiety disorders: Aversive conditioning, context, and neurobiology. Biological psychiatry, 52, 958–975. Grillon, C., Ameli, R., Woods, S., Merikangas, K., & Davis, M. (1991). Fear-potentiated startle in humans: Effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology, 28(5), 588–595. Helmstetter, F. J. (1992). The amygdala is essential for the expression of conditional hypoalgesia. Behavioral neuroscience, 106(3), 518–28. Helmstetter, F. J., & Bellgowan, P. S. (1993). Lesions of the amygdala block conditional hypoalgesia on the tail flick test. Brain research, 612(1-2), 253–257. Helmstetter, F. J., & Bellgowan, P. S. (1994). Effects of muscimol applied to the basolateral amygdala on acquisition and expression of contextual fear conditioning in rats. Behavioral neuroscience, 108(5), 1005–9. Herwig, U., Baumgartner, T., Kaffenberger, T., Brühl, A., Kottlow, M., Schreiter-Gasser, U., Abler, B., et al. (2007). Modulation of anticipatory emotion and perception processing by cognitive control. NeuroImage, 37(2), 652–62. doi:10.1016/j.neuroimage.2007.05.023 Indovina, I., Robbins, T. W., Núñez-Elizalde, A. O., Dunn, B. D., & Bishop, S. J. (2011). Fear-conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron, 69(3), 563–71. doi:10.1016/j.neuron.2010.12.034 129 Jovanovic, T., & Ressler, K. J. (2010). How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. The American journal of psychiatry, 167(6), 648–62. doi:10.1176/appi.ajp.2009.09071074 Kahnt, T., Heinzle, J., Park, S. Q., & Haynes, J.-D. (2011). Decoding different roles for vmPFC and dlPFC in multi-attribute decision making. NeuroImage, 56(2), 709–15. doi:10.1016/j.neuroimage.2010.05.058 Kim, J. J., & Jung, M. W. (2006). Neural circuits and mechanisms involved in Pavlovian fear conditioning: A critical review. Neuroscience & Biobehavioral Reviews, 30(2), 188–202. Kim, M. J., Gee, D. G., Loucks, R. A., Davis, F. C., & Whalen, P. J. (2011). Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cerebral cortex (New York, N.Y. : 1991), 21(7), 1667–73. doi:10.1093/cercor/bhq237 Kimmel, E. (1967). Judgments of UCS intensity and diminution of the UCR in classical GSR conditioning. Journal of experimental psychology, 73, 532–543. Klumpp, H., Ho, S. S., Taylor, S. F., Phan, K. L., Abelson, J. L., & Liberzon, I. (2011). Trait anxiety modulates anterior cingulate activation to threat interference. Depression and anxiety, 28(3), 194–201. doi:10.1002/da.20802 Knight, D. C., Lewis, E. P., & Wood, K. H. (2011). Conditioned diminution of the unconditioned skin conductance response. Behavioral neuroscience, 125(4), 626– 31. doi:10.1037/a0024324 Knight, D. C., Nguyen, H. T., & Bandettini, P. A. (2003). Expression of conditional fear with and without awareness. Proceedings of the National Academy of Sciences of the United States of America, 100(25), 15280–3. doi:10.1073/pnas.2535780100 Knight, D. C., Nguyen, H. T., & Bandettini, P. A. (2005). The role of the human amygdala in the production of conditioned fear responses. NeuroImage, 26(4), 1193–200. doi:10.1016/j.neuroimage.2005.03.020 Knight, D. C., Nguyen, H. T., & Bandettini, P. A. (2006). The role of awareness in delay and trace fear conditioning in humans. Cognitive, affective & behavioral neuroscience, 6(2), 157–62. Knight, D. C., Smith, C. N., Cheng, D. T., Stein, E. A., & Helmstetter, F. J. (2004). Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cognitive, affective & behavioral neuroscience, 4(3), 317–25. 130 Knight, D. C., Smith, C. N., Stein, E. A., & Helmstetter, F. J. (1999). Functional MRI of human Pavlovian fear conditioning: patterns of activation as a function of learning. Neuroreport, 10(17), 3665–70. Knight, D. C., Waters, N. S., King, M. K., & Bandettini, P. A. (2010). Learning-related diminution of unconditioned SCR and fMRI signal responses. NeuroImage, 49(1), 843–8. doi:10.1016/j.neuroimage.2009.07.012 LeDoux, J. (1998). Fear and the brain: Where have we been, and where are we going? Biological psychiatry, 44(12), 1229–38. LeDoux, J. (2003). The emotional brain, fear, and the amygdala. Cellular and molecular neurobiology, 23(4-5), 727–38. LeDoux, J. (2007). The amygdala. Current biology: CB, 17(20), 868–874. Lykken, D. T., Macindoe, I., & Tellegen, A. (1972). Preception: Autonomic Response to Shock as a Function of Predictability in Time and Locus. Psychophysiology, 9(3), 318–333. Maier, S. F., Amat, J., Baratta, M. V, Paul, E., & Watkins, L. R. (2006). Behavioral control, the medial prefrontal cortex, and resilience. Dialogues in Clinical Neuroscience, 8(4), 397–407. Marcos, J. L., & Redondo, J. (1999). Effects of conditioned stimulus presentation on diminution of the unconditioned response in aversive classical conditioning. Biological psychology, 50(2), 89–102. Maren, S. (2001). Neurobiology of Pavlovian fear conditioning. Annual review of neuroscience, 24, 897–931. doi:10.1146/annurev.neuro.24.1.897 Milad, M. R., Pitman, R. K., Ellis, C. B., Gold, A. L., Shin, L. M., Lasko, N. B., Zeidan, M. A., et al. (2009). Neurobiologial basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry, 66(12), 1075–1082. doi:10.1016/j.biopsych.2009.06.026. Milad, M. R., Quirk, G. J., Pitman, R. K., Orr, S. P., Fischl, B., & Rauch, S. L. (2007). A role for the human dorsal anterior cingulate cortex in fear expression. Biological psychiatry, 62(10), 1191–4. doi:10.1016/j.biopsych.2007.04.032 Milad, M. R., Rauch, S. L., Pitman, R. K., & Quirk, G. J. (2006). Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biological psychology, 73(1), 61–71. doi:10.1016/j.biopsycho.2006.01.008 131 Milad, M. R., Vidal-Gonzalez, I., & Quirk, G. J. (2004). Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behavioral neuroscience, 118(2), 389–94. doi:10.1037/0735-7044.118.2.389 Morgane, P. J., Galler, J. R., & Mokler, D. J. (2005). A review of systems and networks of the limbic forebrain/limbic midbrain. Progress in neurobiology, 75(2), 143–60. doi:10.1016/j.pneurobio.2005.01.001 Nitschke, J. B., Sarinopoulos, I., Mackiewicz, K. L., Schaefer, H. S., & Davidson, R. J. (2006). Functional neuroanatomy of aversion and its anticipation. NeuroImage, 29(1), 106–16. doi:10.1016/j.neuroimage.2005.06.068 Nitschke, J. B., Sarinopoulos, I., Oathes, D. J., Johnstone, T., Whalen, P. J., Davidson, R. J., & Kalin, N. H. (2009). Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. The American journal of psychiatry, 166(3), 302–10. doi:10.1176/appi.ajp.2008.07101682 Pavlov, I. (1927). Conditioned reflexes. Oxford University Press, London. Peeke, S. C., & Grings, W. W. (1968). Magnitude of UCR as a function of variability in the CS–UCS relationship. Journal of experimental psychology, 77, 1–6. Phillips, M. L., Drevets, W. C., Rauch, S. L., & Lane, R. (2003). Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biological Psychiatry, 54(5), 504–514. doi:10.1016/S0006-3223(03)00168-9 Rauch, S. L., Shin, L. M., & Phelps, E. A. (2006). Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biological psychiatry, 60(4), 376–82. doi:10.1016/j.biopsych.2006.06.004 Ressler, K. J., Rothbaum, B. O., Tannenbaum, L., & Anderson, P. (2004). Cognitive Enhancers as Adjuncts to Psychotherapy: use of D-Cycloserine in phobic individuals to facilitate extinction of fear. Archives of general psychiatry, 61, 1136-1144. Salomons, T. V, Johnstone, T., Backonja, M.-M., & Davidson, R. J. (2004). Perceived controllability modulates the neural response to pain. The Journal of neuroscience: the official journal of the Society for Neuroscience, 24(32), 7199–203. doi:10.1523/JNEUROSCI.1315-04.2004 Salomons, T. V, Johnstone, T., Backonja, M.-M., Shackman, A. J., & Davidson, R. J. (2007). Individual differences in the effects of perceived controllability on pain perception: critical role of the prefrontal cortex. Journal of cognitive neuroscience, 19(6), 993–1003. doi:10.1162/jocn.2007.19.6.993 132 Schienle, A., Köchel, A., Ebner, F., Reishofer, G., & Schäfer, A. (2010). Neural correlates of intolerance of uncertainty. Neuroscience letters, 479(3), 272–6. doi:10.1016/j.neulet.2010.05.078 Schultz, D. H., & Helmstetter, F. J. (2010). Classical conditioning of autonomic fear responses is independent of contingency awareness. Journal of experimental psychology. Animal behavior processes, 36(4), 495–500. doi:10.1037/a0020263 Sehlmeyer, C., Dannlowski, U., Schöning, S., Kugel, H., Pyka, M., Pfleiderer, B., Zwitserlood, P., et al. (2011). Neural correlates of trait anxiety in fear extinction. Psychological medicine, 41(4), 789–98. doi:10.1017/S0033291710001248 Vervliet, B., Vansteenwegen, D., & Eelen, P. (2004). Generalization of extinguished skin conductance responding in human fear conditioning. Learning & memory, 11(5), 555–8. doi:10.1101/lm.77404 Viinikainen, M., Jääskeläinen, I. P., Alexandrov, Y., Balk, M. H., Autti, T., & Sams, M. (2010). Nonlinear relationship between emotional valence and brain activity: evidence of separate negative and positive valence dimensions. Human brain mapping, 31(7), 1030–40. doi:10.1002/hbm.20915 Volz, K. G., Schubotz, R. I., & Von Cramon, D. Y. (2003). Predicting events of varying probability: uncertainty investigated by fMRI. NeuroImage, 19(2), 271–280. doi:10.1016/S1053-8119(03)00122-8 Wiech, K., Kalisch, R., Weiskopf, N., Pleger, B., Stephan, K. E., & Dolan, R. J. (2006). Anterolateral prefrontal cortex mediates the analgesic effect of expected and perceived control over pain. The Journal of neuroscience: the official journal of the Society for Neuroscience, 26(44), 11501–9. doi:10.1523/JNEUROSCI.2568-06.2006 133 APPENDIX IRB APPROVAL FORM 134 135