* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 15 Viral Vector-Based Techniques for Optogenetic
Premovement neuronal activity wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Neuropsychology wikipedia , lookup
Neuroplasticity wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Subventricular zone wikipedia , lookup
History of neuroimaging wikipedia , lookup
Aging brain wikipedia , lookup
Neuroinformatics wikipedia , lookup
Multielectrode array wikipedia , lookup
Neurogenomics wikipedia , lookup
Haemodynamic response wikipedia , lookup
Neuroeconomics wikipedia , lookup
Synaptic gating wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Neural engineering wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Development of the nervous system wikipedia , lookup
Nervous system network models wikipedia , lookup
Metastability in the brain wikipedia , lookup
Neuroanatomy wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
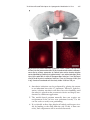
Chapter 15 Viral Vector-Based Techniques for Optogenetic Modulation In Vivo Mathias Mahn, Shiri Ron, and Ofer Yizhar Abstract Optogenetics is a technical methodology that allows direct light-based manipulation of genetically specified cells. Optogenetic methods have provided novel insights into the role of defined neuronal populations in brain function and animal behavior. An expanding palette of single-component optogenetic tools provides powerful interventional strategies for modulating the function of targeted neurons in awake, behaving mammals and for detailed interrogation of circuit physiology in vitro. Although several genetic methods can be utilized for delivering these genes into target cell populations, the use of viral vectors for delivery of optogenetic tools has several important advantages. In recent years, techniques for viral vector-mediated delivery of optogenetic tools have improved and expanded significantly. These techniques now allow modular use of optogenetic tools in defined cell types and circuits and dovetail well with genetic mouse models and recombinase-based driver lines. Here, we review the use of viral vectors for delivering genes encoding optogenetic tools into the rodent brain and provide a detailed protocol for viral transduction of mouse cortical neurons and chronic implantation of a fiberoptic connector for light delivery in vivo. Key words Optogenetics, Lentivirus, Adeno-associated virus, Circuit tracing, Fiberoptic cannula 1 Introduction Microbial rhodopsins are light-sensitive, retinal-containing proteins known for many years to be crucial for the survival and function of a wide range of microbial species [1]. Recently, with the discovery of channelrhodopsin [2, 3] and the first application of this microbial opsin for stimulating neurons [4, 5], neuroscientists began to realize the immense potential of these light-activated proteins to serve as genetically encoded tools for manipulating the activity of defined neural circuit elements with high spatiotemporal resolution [6]. Optogenetics relies on the natural capacity of microbial rhodopsins and other engineered light-responsive Mahn and Ron contributed equally to this work. Riccardo Brambilla (ed.), Viral Vector Approaches in Neurobiology and Brain Diseases, Neuromethods, vol. 82, DOI 10.1007/978-1-62703-610-8_15, © Springer Science+Business Media, LLC 2014 289 290 Mathias Mahn et al. proteins to encode both light sensation and effector function [7]. A major advantage of microbial opsin-based optogenetic tools is that they are essentially cofactor free. The endogenous presence of the organic cofactor all-trans-retinal (ATR) in vertebrate tissues enables the use of these genes as single-component tools, allowing for spatially and temporally precise functional modulation of the intact mammalian nervous system. Neurons transduced with microbial opsin genes become sensitized to light with the physiological effect of illumination dictated by the type of optogenetic tool being used. For example, expression of channelrhodopsin-2 (ChR2), a light-gated cation channel [3, 4], renders cells excitable by light. Light-gated ion pumps such as halorhodopsin (e.g., NpHR) or archaerhodopsin (e.g., Arch) can be used to hyperpolarize and inhibit the electrical activity of neurons [8, 9]. In recent years, the optogenetic toolbox has greatly expanded and now includes multiple tools for excitation, inhibition, and modulation of signal-transduction pathways in neuronal and non-neuronal cells [6]. We and others have reviewed the criteria for selection of optogenetic tools for particular experiment types [7, 10–12]. The choice of opsin for a given experiment will depend on a multitude of factors, including the desired physiological effect, the type of neuron targeted, the spatiotemporal extent of modulation, and the light-delivery system utilized [13]. The use of viral vectors, therefore, provides cost effective and flexible means of optimizing experiment-specific tools for each application. When applied in the adult brain, optogenetics requires genetic modification of post-mitotic neurons to induce expression of the light-gated channels or pumps. Genetically engineered viruses are by far the most popular means of delivering optogenetic tools. Lentiviral vectors (LV) [14] and adeno-associated viral vectors (AAV) [15] have been widely used to introduce opsin genes into mouse, rat, and primate neural tissues [11]. These vectors allow high expression levels over long periods of time with little or no reported adverse effects [15]. AAV-based expression vectors possess lower immunogenicity and offer the advantage of larger transduced tissue volumes compared with LV due to their high viral titers and diffusion properties. Additionally, AAV is considered safer than LV as the currently available strains do not broadly integrate into the host genome and are thus rated as biosafety level (BSL) 2 agents. Both LV and AAV vectors can be used in conjunction with cell type-specific promoters (e.g., [14, 16, 17], see [10] for more examples), and both vector families support pseudotyping techniques, which in principle enable a wide range of cell-type tropisms and transduction mechanisms [18, 19]. Finally, Credependent vectors, in which Cre recombination can activate the expression of transgenes to achieve cell type-specific control [20, 21], are typically made with AAV-based vectors. Viral Vector-Based Techniques for Optogenetic Modulation In Vivo 291 Typical AAV vectors can be produced at much higher titers than LV (typical yields of AAV reach 1013 genome-containing capsids per ml, whereas the same amount of starting material will yield ~109 VSVG-pseudotyped lentivirus). In spite of its huge potential in brain research, several properties of AAV make its production as a recombinant viral vector a technical challenge for most neuroscience laboratories. One such property is the inherent instability of the inverse tandem repeat sequences (ITR’s) required for correct demarcation of the genomic sequence inserted into the capsid, as well as for effective reverse strand synthesis which is a rate limiting step for efficient gene expression from AAV. In addition, in the absence of the full complement of AAV genes and a helper virus (such as adenovirus) in the laboratory setting, recombinant AAV is not exuded from the cells, thus requiring the lysis of the producer cell culture during the purification process. This requires several technically challenging downstream purification steps such as purification columns or ultracentrifugation [22]. Although both LV and AAV have been successfully used in optogenetic experiments, most neuroscience laboratories that do not possess molecular biology and tissue culture capabilities choose to purchase AAV-based vectors from core facilities offering virus production services where commonly used viruses are often kept in-stock as injection-ready aliquots. In optogenetic experiments, several critical factors should be taken into account in order to assure that the desired physiological effect is evoked by light stimulation (1) expression of the optogenetic actuator should be robust and allow modulation with moderate light power in order to avoid phototoxicity; (2) expression should be restricted to the desired neuronal population, with minimal “genetic leak” to nontargeted cell populations; (3) the method used to express the selected tool should be well tolerated and nontoxic to cells over the entire duration of the experimental period (and ideally well beyond this time); (4) in behavioral experiments, the physiological effect of the manipulation performed should always be validated in one of several electrophysiological recording methods to assure that the desired effect is achieved and to correctly interpret behavioral results. For example, acute slice patchclamp recordings can be performed to verify expression and the efficacy of light stimulation; extracellular recordings can further provide important validation of the effect of the optogenetic manipulation in the intact circuit. 1.1 Target Volume Considerations Depending on the scientific question, the required spatial distribution of transduced cells can vary dramatically. This necessitates adjustment of the viral delivery procedure and viral vector type to allow for efficient transduction in the target tissue. Furthermore, the choice of optogenetic tool and illumination method is critical 292 Mathias Mahn et al. to assure that the desired effect is achieved in the targeted cells. The extent of viral transduction depends both on the type of vector used and the brain region targeted. Generally, a relatively restricted expression pattern can be achieved by choosing the appropriate viral vector and injection volume. For example, AAV2 injection results in expression patterns that are more localized compared with the pseudotyped AAV2/5, AAV2/8, or AAV2/9. AAV2 is therefore well suited for local expression in volumes smaller than 1 mm3 [18]. Although viral titer can be reduced in order to decrease the size of the transduced volume, lower titer injections are also likely to influence the number of genome copies in transduced cells, leading to lower expression levels of the transgene in individual cells within the region [18]. On par with AAV2 transduction, LV transduction is more spatially restricted in vivo and can thus be used to target smaller structures. However, LV has been reported to exhibit a bias towards excitatory neurons in cortex [23], an effect which is likely also region specific, since other more specialized cell types have been successfully targeted with lentiviral vectors [24, 25]. If the target area is large, there are several possible limitations to the spatial extent of tissue that can be affected through optogenetic techniques. In the case of optogenetic inhibition, light-gated ion pumps such as NpHR [8] and Arch [9] are commonly used. These opsins have been optimized for neuroscience applications by adding various targeting motifs and are efficiently expressed in neurons [13, 26]. Due to the need for continuous illumination for activation of these ion pumps, the limiting factor in experiments utilizing these tools is the light power density required to achieve effective hyperpolarization of the expressing neurons. Since brain tissue strongly absorbs visible light and is a highly scattering medium, light power density drops to ~1 % within 1 mm from the light source (although this effect is strongly wavelength dependent; see references [9, 10, 24, 27] for more details). The maximal applicable light power for optogenetic modulation is further limited by its cytotoxic effect above certain irradiance levels. A power density of 100 mW/mm2 is regarded as the upper bound for direct light stimulation in brain tissue, thereby restricting the volume of tissue that can be modulated with optogenetic tools requiring intense light for effective modulation (for example, the effective power dose required for eNpHR3.0 is 5 mW/mm2, while most channelrhodopsin variants require less than 1 mW/mm2 [13]). A single injection of 1 μl of AAV2/5 or AAV2/1 can lead to expression in a region >1 mm3 (see Fig. 3 for a representative example). If the opsin expressed is eArch3.0 or eNpHR3.0, the volume affected by light stimulation through an implanted optic fiber in this region is likely to be smaller than the transduced volume [28]. Therefore, in this scenario the transduction volume is normally not restrictive. In the case of neuronal excitation, new tools such as the stepfunction opsins (SFO [29, 30]) can be used to effectively Viral Vector-Based Techniques for Optogenetic Modulation In Vivo 293 depolarize neurons at very low light power densities (up to 3 orders of magnitude lower power density values than required for stimulation with wild-type ChR2). The slow closing kinetics of these channels allows for the accumulation of open channels over long periods of time. This allows for neurons in large volumes of brain tissue to be depolarized with relatively low light power densities, especially if millisecond precise control of neuronal activity is not needed, or even undesired [30]. Here, viral transduction volume can become a limiting factor. The transduction efficiency, titer and binding affinity to the cell surface of the injected viral vector as well as its spread radius can be tailored to the desired outcome, as detailed below. Recent advances in AAV pseudotyping techniques have resulted in strongly enhanced transduction efficiency [31]. AAV2/9 has been shown to possess far superior transduction efficiency compared to wild-type AAV2 ([32, 33]; for review see [34]) and hence results in larger transduction volumes [35]. The distance of viral spread is dependent on multiple factors, including particle size and abundance of the cognate receptor for cell entry. One method to overcome the small range of diffusion-based injection protocols is rapid injection, resulting in convection as shown in 2006 by Raghavan and colleges [36]. This method, referred to as convection-enhanced delivery (CED) seems promising, but requires special injection needles that can prevent viral backflow along the needle insertion tract. Another option is the coadministration of agents aimed at increasing the diffusion of viral particles. Intravenous mannitol injection [37] can be used to decrease intracranial pressure, resulting in increased diffusion distance. A 2 μl injection of AAV2/9, delivered using CED combined with systemic mannitol administration allows for the effective transduction of complete brain regions such as the hippocampus, including contralateral structures [38]. In the case of AAV2, which binds to heparan sulfate proteoglycans on the cell surface, coinfusion of heparin was reported to increase viral spread [39]. Although these advanced methods have not been explored directly for optogenetic experiments, we expect that they will be useful when the targeting of larger volumes is desired. 1.2 Circuit-Based Expression of Optogenetic Tools Functional dissection of intact neural circuits is one of the most widely used applications of optogenetic techniques. Channelrhodopsin can itself serve as a tool for anterograde circuit mapping [40, 41]. Introduction of fluorescently tagged channelrhodopsins into a specific neuronal population in a defined brain region enables the visualization and subsequent photoactivation of projecting axon terminals throughout the brain. Simultaneous electrophysiological recording at the projection site allows the identification of specific postsynaptic components of the circuit both in vivo and in the acute brain slice preparation [40, 42–46]. 294 Mathias Mahn et al. At the whole-brain level, optogenetic stimulation has also been integrated with functional magnetic resonance imaging (ofMRI [47–49]) for identification of global circuits recruited by defined local activity patterns. This method enables unbiased mapping of functionally connected areas without a priori knowledge of circuit connectivity and allows identification of downstream loci. Recently, several new viral techniques have been developed that allow the expression of optogenetic tools in neuronal populations that are defined by their pattern of synaptic connectivity. Integrating these tracing methods with optogenetics allows combined anatomical and functional dissection of defined circuits. Several recent studies have described and optimized an elegant technique for tracing monosynaptic retrograde connections with a modified rabies virus; this approach is based on a glycoprotein-deleted variant of the rabies SAD B19 strain, SADΔG [50, 51]. The rabies virus glycoprotein (G), which is embedded in the viral membrane, is required for trans-synaptic spread. By introducing the glycoprotein gene in neurons prior to infection of the G-deleted mutant virus, the virus spreads to presynaptic neurons and is restricted from further spread due to the lack of this complementary glycoprotein in newly infected neurons [52]. This enables the dissection of direct connections originating from a population of defined neurons or even from a single primary infected neuron [53]. Osakada et al. [54] incorporated ChR2 into the glycoproteindeleted rabies virus (yielding SADΔG-ChR2-mCherry) and successfully displayed optical activation of presynaptic ChR2 expressing cells. This system makes it possible to outline the function of specific connections through combination with electrophysiological recordings or behavioral paradigms. For an additional layer of specificity, the primary viral transduction can be directed to genetically defined post-synaptic neuronal subtypes by using the avian receptor TVA system [55]. To achieve specificity, the SADΔG virus is pseudotyped with an envelope protein from the avian sarcoma and leukosis virus (ASLV). The TVA receptor, which is required for infection by the pseudotyped rabies virus and is only found in birds, is then expressed in the cells to be targeted for infection by SADΔG along with the rabies glycoprotein. This allows the virus to spread trans-synaptically exclusively from the TVA-expressing cells to their presynaptic partners. Specificity in retrograde transport can also be achieved by targeting the rabies G protein gene to specific neurons using the double-floxed Cre-based expression system [56]. In this preparation, only Cre-expressing cells will harbor the necessary machinery for retrograde transport of the recombinant rabies virus. To broaden the microbial opsin repertoire available for monosynaptic retrograde tracing, one could introduce a Cre recombinase-expressing SADΔG virus into transgenic mice engineered for Cre-dependent ChR2/Arch/NpHR expression [57]. The advantages of using rabies-based circuit tracing Viral Vector-Based Techniques for Optogenetic Modulation In Vivo 295 techniques are the unidirectional spread, fast expression, and high amplification capabilities of the SAD B19 virus [58]. On the other hand, the time course of survival of SAD B19-infected neurons is limited with cell health diminishing about 2 weeks following infection [51], suggesting that for experiments requiring long-term survival of transduced neurons, other systems might be required. An additional method for trans-synaptic circuit tracing is the use of Cre-tagged trans-cellular tracer proteins. Trans-cellular tracer proteins can be transported across synapses after binding to specific glycoconjugates on the membrane (reviewed in [59]). When using these proteins to confer trans-synaptic transport function, it is possible to fuse proteins of interest to these tracers, thereby allowing circuit tracing (with fluorescent markers) as well as delivery of a functional protein if desired (such as enzymes). Taking advantage of the trans-cellular transport properties of wheat germ agglutinin (WGA), ChR2 can be expressed in a subset of synaptically connected neurons [26]. This system requires two separate constructs and viral injection sites: the first, a WGA–Cre fusion protein for combined recombinase and trans-synaptic functionality, which can be expressed using AAV and targeted stereotactically to a defined brain region. Cre-dependent AAV vector, conditionally expressing a microbial opsin gene, is then targeted to a predicted trans-synaptic target region. Although this method has been successfully demonstrated in cortico-cortical and hippocampal circuits, the directionality of transport of WGA–Cre has not yet been fully characterized and might well be circuit dependent. In this type of experiment, prior knowledge of synaptic connections between the targeted neuronal populations is necessary in order to determine the secondary injection site. For a more unbiased means of functional mapping, it is possible to introduce WGA–Cre into transgenic mice that conditionally express fluorescent reporter proteins [60] or microbial opsins [57]. Although several retrograde trans-synaptic tracing approaches have been described, anterograde-specific tracer viruses are not yet sufficiently optimized for optogenetic experiments. Currently, the most promising viral tool for anterograde tracing is the H129 strain of herpes simplex virus (HSV) type 1 [61] which has recently been developed for genetically defined, Cre-dependent, anterograde tracing [62]. A possible application of this technology would be the insertion of Cre-dependent microbial opsins into the H129 genome enabling light-induced activation of downstream circuit elements. A different strain of HSV1, which is taken up by axons and transported retrogradely, has been used for expression of ChR2 in neurons projecting to the site of injection. This allows for photostimulation-assisted identification of neuronal populations (PINP [63]). Importantly, not all HSV viruses spread anterogradely, and the directionality depends on the specific strain used. As with many other neurotropic viral vectors, the main 296 Mathias Mahn et al. disadvantage of this viral system is its adverse effect on cell and animal health over a course of days and weeks. 1.3 2 2.1 Summary Viral vector-based expression is a versatile and widely used method for delivering optogenetic tools in the brain. However, animal-toanimal variation in the efficiency of viral transduction is also a potential major source of variability in optogenetic experiments. Although electrophysiology and immunohistochemistry can allow researchers to correlate the behavioral or physiological effect with the efficiency of genetic expression of the optogenetic tool, optimization of the method for viral transduction and light delivery to the targeted cells can reduce this inherent variability and allow more robust, reproducible results. In the protocol below, we focus on the procedures developed for direct intracerebral delivery of high-titer virus for expression of optogenetic tools in defined cell populations. We also describe the procedure for chronic implantation of fiberoptic connectors for robust, reproducible optical modulation of virally transduced neurons in vivo. Protocol Reagents Iodine antiseptic (e.g., Betadine) Ethanol, 70 % Sterile saline Sterile distilled, deionized water (DDW) Anesthetics and analgesics Lubricant eye ointment Optional: hydrogen peroxide (H2O2) 2.2 Equipment 2.2.1 Virus Injection Surgical tools (e.g., curved sharp tip tweezers; fine, spring scissors; surgical scalpel; dull-tip forceps; wide tipped bulldog clamps) Hot bead tool sterilizer (see Note 1) Dissection stereomicroscope (Fig. 1-a1) Small animal stereotaxic apparatus with cannula holder, nonrupture tip ear-bars, tooth bar, rodent face mask for isoflurane circulation (Fig. 1-a2, a3) Temperature-controlled heating pad (Fig. 1-a4, a5) Isoflurane vaporizer (LEI Medical; Fig. 1-a6) Isoflurane induction box (Fig. 1-a7) Small heat lamp Small hair trimmer Viral Vector-Based Techniques for Optogenetic Modulation In Vivo 297 Fig. 1 Surgery setup and procedure. (a) Overview of the surgery setup including (1) stereomicroscope, (2) stereotaxic instrument, (3) display of stereotaxic coordinates, (4) mouse stretcher with temperature controlled heating pad, (5) temperature controller, (6) isoflurane vaporizer, (7) isoflurane induction box. (b) Major steps of viral vector injection procedure: (I) head fixation with tooth bar, face mask, and ear bars, (II) Scalp incision and skull exposure using wide-tipped bulldog clamps, (III) axis zeroing with needle tip at bregma, (IV) head orientation by horizontal and vertical lambda alignment, (V) marking of the intended hole position, (VI) needle insertion into the brain and injection. (c) Major steps of optical fiber implantation: (I) shortening of the optical fiber to required length using the ear bar scale, (II) axis zeroing with optic fiber tip at bregma, (III) placement of the optic fiber above the injection site, (IV) adhesion enhancement by application of Metabond, (V) fixation of the optic fiber to the skull using dental acrylic, (VI) closing of the incision around the dental acrylic using tissue adhesive Micro motor drill with micro burrs (0.5–0.7 mm) 1 ml syringe with needles (30G) for intraperitoneal injections Microsyringe pump (e.g., World Precision Instruments UMP-4) Cotton swabs, optional: absorbent swabs (Sugi) 50 ml conical tubes Vetbond tissue adhesive or surgical suture 298 Mathias Mahn et al. 2.2.2 Fiber Implants Implantable fiberoptic connector with metal or ceramic ferrule— fiber width: 200 μm diameter; fiber length: according to injection site DV coordinate (Fig. 1-bI) Cannula holder for stereotactic frame C&B Metabond kit Acrylic dental cement 2.2.3 Behavioral Testing Open field chamber for mice: square arena 50 cm × 50 cm made of gray plastic with 40 cm high walls. Mouse behavioral tracking system (Viewer3, BIOBSERVE GmbH, St. Augustin, Germany) Master-8 pattern generator (A.M.P.I, Jerusalem, Israel) 447 nm, 100 mW diode laser (O.E.M Laser Systems Inc., Salt Lake City, UT, USA) Fiberoptic patch-cord (200 μm core, Thorlabs Inc., cat # M72L05) Fiberoptic patch-cord (200 μm core, FC/PC to bare ferrule connector, Doric Lenses, Quebec, Canada) Fiberoptic rotary joint (Doric Lenses; Cat. # FRJ 1X1 FC/FC) Zirconia sleeve for ferrule–ferrule connection (Thorlabs Inc., cat # ADAF1) 2.3 Setup 1. Viral vectors ● ● Storage: viruses should be stored in aliquots of 5–10 μl at −80 °C. Thawing: on day of surgery, virus aliquots should be thawed quickly in a gloved hand or a warm water bath and then kept at 4 °C until use. AAV can be stored after thawing for several days at 4 °C with minimal loss of activity. LV is less stable and should be used on the same day. 2. Preparation of surgical tools ● Set heat bead sterilizer to 250 °C. ● Sterilize surgical tools by placing them, for at least 45 s, in sterilizer so that the tips are fully covered by the beads. ● Arrange the sterilized surgical tools on a sterile surgical pad next to the stereotaxic instrument and allow them to cool before beginning the procedure. 3. Preparation of the viral injection syringe ● The syringe should be washed thoroughly with DDW and then sterile saline prior to virus uptake. This is a good opportunity to make sure that the needle is not clogged by visually confirming that liquid is withdrawn and expelled by slowly moving the syringe plunger up and down. Viral Vector-Based Techniques for Optogenetic Modulation In Vivo 299 4. Coordinates ● Coordinates of the different brain regions can be determined using brain atlases [64] (see Note 2). Coordinates of the intended injection area are given in three axes: X: medial–lateral (ML), Y: anterior–posterior (AP), and Z: dorsal–ventral (DV). 5. Anesthetics and analgesics: in this protocol, we describe the use of isoflurane for anesthesia, but other methods are also possible (see Note 3). 2.4 Surgical Procedure ● Induction: We suggest using a ketamine (80 mg/kg) xylazine (10 mg/kg) mix for anesthesia induction (see Note 4). To prevent dehydration of the mouse during surgery, it is recommended to administer 500 μl saline subcutaneously during the procedure. ● At least 30 min before the end of surgery 0.05 mg/kg buprenorphine (or any other approved analgesic) should be injected for postsurgical analgesia. Surgeries must be performed in accordance with regulations and should be conducted in clean conditions. For proper handling of animals and recommended materials please refer to the NIH guidelines (http://www.oacu.od.nih.gov/ARAC/documents/Rodent_ Surgery.pdf). It is recommended to use a stereomicroscope throughout the procedure for accuracy and reproducibility. It is crucial to verify the depth of anesthesia by monitoring breathing rate and toe-pinch reflex throughout the surgery at intervals of 10–15 min. Isoflurane clearance should be managed according to local regulations, either through passive or active removal from the mask. 1. Weigh the mouse and calculate the appropriate dose of preanesthesia (optional) and analgesia. Administer pre-anesthesia or place the mouse in the isoflurane induction chamber. 2. Shave fur on mouse head using scissors or an electric hair trimmer. Trim from back of skull to slightly posterior to eyes. 3. Apply a layer of eye ointment over the whole eye area, to avoid corneal drying during the surgery. 4. Place mouse in the animal stereotaxic apparatus: ● Pull back skin on head and pull down bottom jaw. ● Position head so that the upper teeth go through the slot of the tooth bar. Pull the mouse gently backward to make sure mouth is fastened correctly in tooth bar. ● Position the isoflurane mask and tighten. 300 Mathias Mahn et al. 5. Activate the isoflurane vaporizer. First adjust to 2 %, then maintain at a working concentration of 1–1.5 % throughout the procedure. 6. Monitor breathing and check toe-pinch reflex: ● Monitoring of breathing and toe-pinch reflex should be done throughout the surgery. Toe-pinch reflex should be checked every 10 min but should be omitted when the injection needle is in the brain (see below). 7. Head-fix mouse: ● Position ear bars: Bars should be positioned either inside the ear canal or slightly ventral and anterior to it, where jaw meets skull. For ideal positioning, both bars should be pushed against the skull simultaneously and from the same starting position (see Note 5; Fig. 1-bI) ● Adjust the height of the tooth bar and/or ear bars in order to achieve correct placement of the skull with respect to bars (see Note 6). ● Once correct position has been obtained, tighten ear bars and make sure the head is fixed tightly by gently applying pressure to the head in a downward direction. 8. Heating pad: ● Heating pad should be placed under the mouse and set to 37 °C. ● Body temperature should be monitored using a rectal probe. ! Make sure heating pad does not overheat. 9. Sterilize scalp: ● Use sterile cotton swabs to wash with iodine antiseptic, then with 70 % ethanol. Repeat this step twice. ● Stroke swab from center of the skull towards the sides. 10. Make incision: ● Using fine-tipped tweezers pull a bit of skin upwards from skull between the ears of mouse and make a small horizontal incision with the scissors, making sure not to damage other tissues. ● Insert scissor tips through the cut so scissors are perpendicular to the skull and facing forward. Cut along the midline until incision reaches between the eyes. ● Use the bulldog clamps to clamp the skin on each side of the incision and move laterally to reveal the entire skull (Fig. 1-bII) Viral Vector-Based Techniques for Optogenetic Modulation In Vivo 301 ● Using a cotton swab, gently swab the entire skull to remove conjunctive tissue. ● Place syringe in stereotactic device: – Insert the injection syringe into its holder, making sure that the plunger is straight and fastened. – If needle is beveled, rotate syringe so that the opening faces the axis that will allow the virus to spread to target area most effectively. Example: bevel could be facing anterior if injection coordinate is relatively at the posterior end of the intended area. – Lower the syringe plunger until base of plunger is close to the bottom of the syringe. 11. Identify bregma (Fig. 1-bIII): ● Identify the anterior part of the skull where the coronal and sagittal sutures intersect (see Note 7). ● Place syringe needle over bregma, touch lightly so as not to bend the needle. ● Zero coordinates on stereotactic device. 12. Identify lambda (Fig. 1-bIV): ● Identify lambda as the imaginary point where the sagittal suture would meet the lambdoid suture if it were a straight line across (typically about 1 mm posterior to the actual intersection point; see [64]). ● Position syringe over lambda, touch lightly. ● Lambda should fall on the same medial–lateral and dorsal– ventral position as bregma. ● Deviations in height between bregma and lambda should not exceed 0.1 mm (see Note 8). 13. Place syringe over injection target AP and ML coordinates and lower until lightly touching skull with needle (Fig. 1-bV). Mark target with surgical marker if desired in order to identify the area for drilling. 14. Craniotomy: ● Using the micro-motor drill, make a craniotomy over the target area by applying light pressure until the dura is visible. Manage bleeding using a cotton swab or Sugi absorbent spear. ● Using fine tweezers or a needle with a bent tip, carefully remove the dura from underneath the craniotomy without damaging the underlying cortex. 302 Mathias Mahn et al. 15. Injection: virus withdrawal: ! ● Precaution: Any object that has contacted the virus suspension is considered biohazardous material and must be treated according to the existing regulations. Such material can be placed in a designated biohazards vial containing ethanol (70 %) or H2O2. Using the syringe, withdraw the virus suspension at a rate of 20–50 nl/s. It is recommended to withdraw at least 1.5 times the exact volume needed in order to ensure sufficient suspension. 16. Injection: preparation step: ! This stage is important to perform in order to assure that no air remains at the needle tip after virus withdrawal. ● While syringe needle is still in the air, infuse until a drop forms at the needle tip. ● Use Sugi tip to absorb the drop and discard into biohazard vial. 17. Injection: Infusion: ! At this stage, movement of the mouse, stereotax and table should be minimized since any excessive vibration could cause movement of the needle inside the brain tissue and leakage of virus through the gap formed by this movement. Do not perform toe-pinch reflex tests until the syringe is retracted from the brain and minimize any movements of the table. ● Recommended: Before injecting, verify the position of bregma and reset the stereotactic coordinates to this location since the skull or needle may have shifted in previous steps. ● Insert syringe through the drilled hole (while confirming correct AP and ML coordinates) and into the brain until reaching the desired DV coordinate. This should be done slowly once the brain is entered (at the rate of about 2 mm/min) for minimal tissue damage (Fig. 1-bVI). ● Wait about 5 min for brain tissue to adjust to needle at coordinate. ● Set infusion speed to 100 nl/min and begin infusion. ● Monitor infusion: If fluid is observed to accumulate at the surface, stop injection, wait several minutes, and/or lower the needle by several tens of microns before continuing with infusion. ● After infusion, wait 10 min for virus to diffuse away from injection site. ● Withdraw syringe slowly (~1 mm/min). Viral Vector-Based Techniques for Optogenetic Modulation In Vivo 303 18. Fiber optic implant: ● The implantable fiberoptic lightguide (IFL) can either be inserted into the injection area or into a predicted projection area of that region [10]. The IFL we implant has a metal or ceramic ferrule adapter which allows for optimal optical coupling between the fiberoptic patch-cord and the implanted fiber. ● The length of the fiber below the ferrule should be about equal to the dorsoventral position of the target. It is recommended to cut the fiber using a diamond knife: score the optic fiber with the diamond knife at the desired length, lay the fiber on a straight surface, and tape down its tip. Holding the ferrule, pull lightly away from taped tip. The fiber should break at the scoreline (Fig. 1-cI). ● Take into consideration that the tip of the fiber should be placed about 0.5 mm above the injection site in order to be able to illuminate maximum volume of the virally-transduced area. ● Replace the syringe holder arm with a cannula holder. ● Place the IFL in the cannula holder so that the fiber tip is pointing down towards the mouse head. Place the IFL over bregma and reset the coordinates on the stereotax (Fig. 1-cII). ● Move the IFL to the target AP and ML coordinates. If the fiber implant area is the same as the injection site, the fiber should be above the previously drilled craniotomy (Fig. 1-cIII). If the fiber implant area is different, proceed with drilling as mentioned above. ● Lower the IFL slowly to the DV coordinate. Make sure that the dura mater has been fully removed from the area before lowering the fiber. ● Once the IFL has been inserted into the correct coordinates, proceed with cementing. It is important not to move the cannula guide yet. 19. Fix optic fiber implant to skull. ● Apply a layer of C&B metabond over the entire surface of the skull, avoiding the craniotomy region (see Note 9; Fig. 1-cIV). ● Allow metabond to dry (5–10 min). ● Apply a few thick layers of dental cement on the metabond and around the fiber and ferrule while leaving at least 5 mm from the top of the ferrule exposed (Fig. 1-cV). This is important in order to ensure sufficient space for the zirconia sleeve that will be attached to this area during the experiments. 304 Mathias Mahn et al. ● Allow cement to dry (5–10 min). ● Slowly raise the cannula holder while pressing down on the dried cement with tweezers. ● The secured ferrule should keep in place, while the cannula holder is being raised. 20. Closing incision: ● With tweezers, press skin together and apply a drop of Vetbond™ tissue glue onto the subcutaneous tissue. Start posterior and progress anteriorly along the incision (Fig. 1-cVI). ● Alternatively, use surgical suture. Typically one suture is done anterior to the implant and another one or two posterior. 21. Remove mouse from stereotactic device and place in a separate cage with fresh bedding and heated by a heat lamp. ● If using isoflurane anesthesia: Before removing mouse, remove ear bars, turn off isoflurane vaporizer, wait 1–2 min, turn off O2, then turn off active isoflurane clearance system (if used). ● Following long surgical procedures, it is recommended to leave mouse with O2 ventilation through the anesthesia mask until detecting a toe pinch reflex. 22. Wash syringe: 2.5 Optogenetic Behavioral Testing ● Withdraw a small volume of saline into the virus-containing syringe. ● Eject virus and saline from syringe into biohazard vial. Do not touch syringe tip to ethanol. ● Fill and empty the syringe three times with DDW. Repeat this step several times with clean DDW to assure effective removal of virus from needle. Dispose of DDW according to biohazard regulations. Behavioral testing can be initiated 2–4 weeks following virus injection, depending on the type of virus and its titer. If light stimulation is targeted at distal axons of the expressing cells, additional time might be required for trafficking of the opsin to the axons. Histological examination can be performed to evaluate the time required for expression and trafficking of the optogenetic proteins in the chosen paradigm. Here we briefly describe a behavioral paradigm used for evaluating the effects of optogenetic activation of the primary mouse motor cortex following AAV-based expression of ChR2(H134R)-mCherry in this region. The mouse used for Viral Vector-Based Techniques for Optogenetic Modulation In Vivo 305 this behavioral experiment was treated as described above and injected with 1 μl AAV2/5-CaMKIIα-ChR2(H134R)-mCherry in M1 cortex [AP 1 mm; ML 1 mm (left); DV 1.5 mm]. The procedure detailed below was performed 4 weeks after injection. 2.6 Procedure 1. Position the rotary joint above the center of the open field chamber and next to the video tracking camera. 2. Connect the long (5 m) patch cord to the laser fiber coupler on one end and to the rotary joint at the other end. 3. Connect the second fiberoptic patch-cord from the rotary joint so that the bare ferrule side terminates at the level of the open field chamber. 4. Remove the mouse from the home cage and attach the fiberoptic patch-cord ferrule to the IFL. 5. Place the mouse in the center of the open field and initiate the behavioral recording session. 6. At defined times, light can be delivered through the fiberoptic cables by triggering the laser through TTL pulses from the pattern generator. Depending on the laser type, digital or analog modulation can be applied to control the light power output (see Note 10). 7. At the end of the experimental session, remove the mouse from the arena, disconnect the fiberoptic patch-cord from the IFL, and return the mouse to the home cage. 2.7 Summary and Expected Results In the protocol section, we describe a procedure for viral transduction of primary motor cortex pyramidal neurons with ChR2(H134R)-mCherry, followed by an optical fiber implantation for in vivo modulation of neuronal activity. To test the effect of optogenetic activation of primary motor cortex pyramidal neurons in a freely moving mouse, we placed the implanted mouse in an open field chamber and recorded the position of the mouse during a behavioral session that lasted 30 min. At defined times, we applied 447 nm light at different frequencies and analyzed the changes in the direction of locomotion compared with baseline (light-off) periods. The raw tracking data were imported into Matlab and analyzed offline for the direction of locomotion. The position of the mouse was sampled in 0.5 s intervals. We measured the angle of the third point relative to the line defined by the first and second points. The resulting vector showed an average angle of 28° towards the contralateral side during episodes of optical stimulation (with respect to the site of optical stimulation). While the angle of this trajectory is clearly dependent on the sampling frequency, the resulting estimated average radius of 6.3 cm is stable over a wide range of sampling intervals. We demonstrate that 306 Mathias Mahn et al. Fig. 2 Optogenetic stimulation of virally transduced motor cortex. (a) Mouse implanted with fiberoptic connector, after recovery from surgery. Fiberoptic patch-cable is attached to the implant, allowing for light delivery into the targeted region. (b) Overlay image indicating the position of the mouse before light stimulation (1), during stimulation (2–5) and after stimulation (6). Stimulation was performed using a 447 nm diode laser at a frequency of 13 Hz and pulse width of 5 ms for 6 s intervals. (c) Radial plot depicting the average speed and direction of movement of the mouse during no-stimulation trials (black) compared with stimulation trials (blue). The mouse’s body position was tracked and the average direction of motion was calculated at 2 Hz sampling intervals. The vector length, representing mouse velocity, was normalized to control periods activation of the left primary motor cortex leads to a bias in the average direction of locomotion toward the right, resulting in circular walking trajectories (Fig. 2). Following behavioral testing we verified the site of injection and observed substantial transduction in the targeted region (Fig. 3). 3 Notes 1. Alternatively, tools can be sterilized by autoclaving or soaking in disinfectant. 2. Brain atlases are usually of adult mice (8 weeks or older) and variations can arise from using different strains and ages. If mice are younger, appropriate adjustments to the target coordinates should be made. The average lambda–bregma distance in C57BL/6J mice, reported in [64], is 4.21 mm. A scaling factor for the stereotaxic coordinates can be obtained by dividing the actual distance in the given mouse by this value. 3. This protocol uses isoflurane for anesthesia and buprenorphine for analgesia. Alternatively, ketamine and xylazine or any other suitable and approved anesthetic can be used. Viral Vector-Based Techniques for Optogenetic Modulation In Vivo 307 Fig. 3 Expression pattern following 1 μl injection of AAV5-CaMKIIα-ChR2(H134R)mCherry into the mouse motor cortex. Virally transduced cells exhibit red fluorescence due to mCherry expression. (a) Coronal brain section showing injection center (identified by injection tract) approximately 1 mm anterior to bregma. Brain injury by the optic fiber is visible at the upper edge. Scale bar: 2 mm. (b) Dorsal view of the brain prior to sectioning. Scale bar: 2 mm. (c) Close up of cells [box in (a)]. Somata of transduced cells are clearly visible. Scale bar: 200 μm 4. Anesthesia induction can be performed by placing the mouse in an induction box with 4 % isoflurane. However, light ketamine/xylazine anesthesia will allow for easier handling until the mouse is placed on the stereotactic device and deeply anesthetized by isoflurane application. 5. The medial–lateral position normally does not require any readjustment if the ear bars were positioned evenly. Use the ear bar scales to verify even positioning. 6. It is advisable to first align height of lambda and bregma visually by looking at the skull from the side. If this is done correctly, little adjustment will be needed afterwards. 308 Mathias Mahn et al. 7. If difficult to detect, it is possible to apply a bit of pressure with tweezers on one of the skull bones in order to better emphasize the sutures under the stereomicroscope. Another possibility is to apply a bit of sterile 4 % H2O2 with a cotton swab. After several seconds, wash the skull with sterile saline to stop the reaction. Suture lines should become more visible after ~10 s of treatment. 8. If necessary, adjust skull position to align lambda and bregma. The medial-lateral position can be adjusted by moving the ear bars simultaneously without releasing the pressure on the skull. If lambda is too high or too low relative to bregma, adjust the mouse’s snout by raising/lowering the tooth bar. 9. Metabond acts as a bonding material between the scalp and the dental cement. A thick layer should be spread on the scalp around the implanted fiber and around the bottom part of the ferrule. Contact between metabond and the exposed craniotomy can be eliminated by applying a layer of aqueous gel or petroleum jelly (Vaseline) into the craniotomy prior to insertion of the IFL. 10. It is advisable to measure the light power output of the optical system by using a portable light power meter (e.g., Thorlabs P-1000D). This can be done using an IFL similar to the implanted one, in order to account for light power losses at all points of optical coupling in the path. Acknowledgments We thank the entire Yizhar lab for helpful comments and discussions on the manuscript and protocol. This work was supported by grants from the Israeli Science Foundation (ISF grant 1351/12) and by the Israeli Center of Research Excellence (I-CORE) in Cognition (I-CORE Program 51/11). Ofer Yizhar is the incumbent of the Gertrude and Philip Nollman Career Development Chair. References 1. Spudich JL, Yang CS, Jung KH, Spudich EN (2000) Retinylidene proteins: structures and functions from archaea to humans. Annu Rev Cell Dev Biol 16:365–392 2. Nagel G et al (2002) Channelrhodopsin-1: a light-gated proton channel in green algae. Science 296:2395–2398 3. Nagel G et al (2003) Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci USA 100:13940–13945 4. Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K (2005) Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8:1263–1268 5. Li X et al (2005) Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc Natl Acad Sci USA 102:17816–17821 6. Deisseroth K (2011) Optogenetics. Nat Methods 8:26–29 Viral Vector-Based Techniques for Optogenetic Modulation In Vivo 7. Zhang F et al (2011) The microbial opsin family of optogenetic tools. Cell 147: 1446–1457 8. Zhang F et al (2007) Multimodal fast optical interrogation of neural circuitry. Nature 446:633–642 9. Chow BY et al (2010) High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 463: 98–102 10. Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K (2011) Optogenetics in neural systems. Neuron 71:9–34 11. Fenno L, Yizhar O, Deisseroth K (2011) The development and application of optogenetics. Annu Rev Neurosci 34:389–412 12. Kravitz AV, Kreitzer AC (2011) Optogenetic manipulation of neural circuitry in vivo. Curr Opin Neurobiol 21:433–439, available at http:// www.ncbi.nlm.nih.gov/pubmed/21420852 13. Mattis J et al (2011) Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat Methods 9:159–172 14. Dittgen T et al (2004) Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proc Natl Acad Sci USA 101:18206–18211 15. Monahan PE, Samulski RJ (2000) Adenoassociated virus vectors for gene therapy: more pros than cons? Mol Med Today 6:433–440 16. Nathanson JL et al (2009) Short promoters in viral vectors drive selective expression in mammalian inhibitory neurons, but do not restrict activity to specific inhibitory cell-types. Front Neural Circuits 3:19 17. Knobloch HS et al (2012) Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73:553–566 18. Burger C et al (2004) Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther 10:302–317 19. Kato S et al (2011) Neuron-specific gene transfer through retrograde transport of lentiviral vector pseudotyped with a novel type of fusion envelope glycoprotein. Hum Gene Ther 22:1511–1523 20. Sohal VS, Zhang F, Yizhar O, Deisseroth K (2009) Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459:698–702 21. Tsai H-C et al (2009) Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324:1080–1084 22. Zolotukhin S et al (2002) Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods 28:158–167 309 23. Nathanson JL, Yanagawa Y, Obata K, Callaway EM (2009) Preferential labeling of inhibitory and excitatory cortical neurons by endogenous tropism of adeno-associated virus and lentivirus vectors. Neuroscience 161:441–450 24. Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L (2007) Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 450:420–424 25. Benzekhroufa K, Liu B-H, Teschemacher AG, Kasparov S (2009) Targeting central serotonergic neurons with lentiviral vectors based on a transcriptional amplification strategy. Gene Ther 16:681–688 26. Gradinaru V et al (2010) Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141:154–165 27. Aravanis AM et al (2007) An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng 4:S143–S156 28. Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K (2009) Optical deconstruction of parkinsonian neural circuitry. Science 324:354–359 29. Berndt A, Yizhar O, Gunaydin LA, Hegemann P, Deisseroth K (2009) Bi-stable neural state switches. Nat Neurosci 12:229–234 30. Yizhar O et al (2011) Neocortical excitation/ inhibition balance in information processing and social dysfunction. Nature 477:171–178 31. Grimm D, Kay MA (2003) From virus evolution to vector revolution: use of naturally occurring serotypes of adeno-associated virus (AAV) as novel vectors for human gene therapy. Curr Gene Ther 3:281–304 32. Duque S et al (2009) Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol Ther 17:1187–1196 33. Foust K et al (2009) Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol 27:59–65 34. Dayton R, Wang D, Klein R (2012) The advent of AAV9 expands applications for brain and spinal cord gene delivery. Expert Opin Biol Ther 12:757–766 35. Cearley CN, Wolfe JH (2007) A single injection of an adeno-associated virus vector into nuclei with divergent connections results in widespread vector distribution in the brain and global correction of a neurogenetic disease. J Neurosci 27:9928–9940 36. Raghavan R et al (2006) Convection-enhanced delivery of therapeutics for brain disease, and its optimization. Neurosurg Focus 20:E12. doi:10.3171/foc.2006.20.4.7 37. Mastakov M, Baer K, Xu R, Fitzsimons H, During M (2001) Combined injection of 310 38. 39. 40. 41. 42. 43. 44. 45. 46. 47. 48. 49. 50. Mathias Mahn et al. rAAV with mannitol enhances gene expression in the rat brain. Mol Ther 3:225–232 Carty N et al (2010) Convection-enhanced delivery and systemic mannitol increase gene product distribution of AAV vectors 5, 8, and 9 and increase gene product in the adult mouse brain. J Neurosci Methods 194:144–153 Nguyen JB, Sanchez-Pernaute R, Cunningham J, Bankiewicz K (2001) Convection-enhanced delivery of AAV-2 combined with heparin increases TK gene transfer in the rat brain. Neuroreport 12:1961–1964 Petreanu L, Huber D, Sobczyk A, Svoboda K (2007) Channelrhodopsin-2 – assisted circuit mapping of long-range callosal projections. Nat Neurosci 10:663–668 Arenkiel BR et al (2007) In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 54: 205–218 Cruikshank SJ, Urabe H, Nurmikko AV, Connors BW (2010) Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron 65:230–245 Atasoy D, Betley JN, Su HH, Sternson SM (2012) Deconstruction of a neural circuit for hunger. Nature 488:172–177. doi:10.1038/ nature11270 Haubensak W et al (2010) Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468:270–276 Stuber GD et al (2011) Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475: 377–380 Tye KM et al (2011) Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471:358–362 Lee JH et al (2010) Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 465:788–792 Kahn I et al (2011) Characterization of the functional MRI response temporal linearity via optical control of neocortical pyramidal neurons. J Neurosci 31:15086–15091 Desai M et al (2011) Mapping brain networks in awake mice using combined optical neural control and fMRI. J Neurophysiol 105:1393–1405 Etessami R et al (2000) Spread and pathogenic characteristics of a G-deficient rabies virus recombinant: an in vitro and in vivo study. J Gen Virol 81:2147–2153 51. Wickersham IR, Finke S, Conzelmann K, Callaway EM (2007) Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat Methods 4:2006–2008 52. Miyamichi K et al (2011) Cortical representations of olfactory input by trans-synaptic tracing. Nature 472:191–196 53. Marshel JH, Mori T, Nielsen KJ, Callaway EM (2010) Targeting single neuronal networks for gene expression and cell labeling in vivo. Neuron 67:562–574 54. Osakada F et al (2011) New rabies virus variants for monitoring and manipulating activity and gene expression in defined neural circuits. Neuron 71:617–631 55. Wickersham IR et al (2007) Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron 53:639–647 56. Lammel S et al (2012) Input-specific control of reward and aversion in the ventral tegmental area. Nature 491:212–217 57. Madisen L et al (2012) A toolbox of Credependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci 15:793–802 58. Callaway EM (2008) Transneuronal circuit tracing with neurotropic viruses. Curr Opin Neurobiol 18:617–623 59. Köbbert C et al (2000) Current concepts in neuroanatomical tracing. Prog Neurobiol 62:327–351 60. Madisen L et al (2010) A robust and highthroughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13:133–140 61. Zemanick MC, Strick PL, Dix RD (1991) Direction of transneuronal transport of herpes simplex virus 1 in the primate motor system is strain-dependent. Proc Natl Acad Sci USA 88:8048–8051 62. Lo L, Anderson DJ (2011) A Cre-dependent, anterograde transsynaptic viral tracer for mapping output pathways of genetically marked neurons. Neuron 72:938–950 63. Lima SQ, Hromádka T, Znamenskiy P, Zador AM, Nitabach MN (2009) PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PLoS One 4:e6099 64. Paxinos G, Franklin KBJ (2004) The mouse brain in stereotaxic coordinates. Gulf Professional Publishing, Boston, MA, p 100