* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Dopamine Modulates the Function of Group II and Group III
Nonsynaptic plasticity wikipedia , lookup
End-plate potential wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Neuroeconomics wikipedia , lookup
Nervous system network models wikipedia , lookup
Time perception wikipedia , lookup
Axon guidance wikipedia , lookup
Neuroanatomy wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Central pattern generator wikipedia , lookup
Aging brain wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Optogenetics wikipedia , lookup
Long-term depression wikipedia , lookup
NMDA receptor wikipedia , lookup
Spike-and-wave wikipedia , lookup
Signal transduction wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Neuromuscular junction wikipedia , lookup
Synaptic gating wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Basal ganglia wikipedia , lookup
Synaptogenesis wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Chemical synapse wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Neurotransmitter wikipedia , lookup
Substantia nigra wikipedia , lookup
Molecular neuroscience wikipedia , lookup
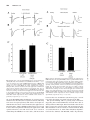
0022-3565/02/3022-433–441$7.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Copyright © 2002 by The American Society for Pharmacology and Experimental Therapeutics JPET 302:433–441, 2002 Vol. 302, No. 2 33266/995631 Printed in U.S.A. Dopamine Modulates the Function of Group II and Group III Metabotropic Glutamate Receptors in the Substantia Nigra Pars Reticulata MARION WITTMANN, MICHAEL J. MARINO, and P. JEFFREY CONN Department of Pharmacology, Emory University School of Medicine, Atlanta, Georgia (M.W., M.J.M., P.J.C.); Department of Neuroscience, Merck Research Laboratories, West Point, Pennsylvania (M.J.M., P.J.C.); and Tierphysiologie, University of Tuebingen, Germany (M.W.) Received February 6, 2002; accepted April 5, 2002 According to current models of basal ganglia (BG) function, DA modulates BG output through differential regulation of medium spiny striatal neurons. These output neurons of the striatum form two separate but parallel descending pathways, the so-called direct and indirect pathways. The direct pathway provides an inhibitory input to the GABA-containing projection neurons of the SNr and the entopeduncular nucleus, the two principal output nuclei of the BG (Grofova et This work was supported by grants from the National Institutes of Health, the National Institute of Neurological Disorders and Stroke, the National Parkinson’s Foundation, the Tourette’s Syndrome Association, National Alliance for Research on Schizophrenia and Depression, and the U.S. Army Medical Research and Material Command. Article, publication date, and citation information can be found at http://jpet.aspetjournals.org. DOI: 10.1124/jpet.102.033266. III mGluR activation [L-(⫹)-2-amino-4-phosphonobutyric acid, L-AP4, 500 M], at STN-SNr synapses is significantly decreased. This effect could be mimicked in control slices by prior bath application of haloperidol (20 M) and R-(⫹)-7-chloro-8hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine (SCH23390) (20 M) but not sulpiride (50 M). Furthermore, application of dopamine (100 M) and (⫾)-6-chloro-7,8dyhydroxy-3allyl-1-phenyl-2,3,4,5-tetra-hydro-1Hbenzazepine (SKF82958) (1 M) but not quinpirole (10 M) could rescue the group II mGluR effect in reserpinized slices. The effect of group III mGluR activation (L-AP4, 100 M) on inhibitory synaptic transmission was also significantly reduced in slices from reserpine-treated animals. This effect was mimicked by haloperidol (20 M), SCH23390 (20 M), and sulpiride (50 M) in control slices. Thus, in a Parkinsonian state, the loss of nigral DA may add to the overall pathophysiological changes in basal ganglia output. al., 1982). The indirect pathway provides a disynaptic disinhibition of the glutamatergic neurons of the subthalamic nucleus (STN), which in turn provides an excitatory input to the BG output nuclei. The balance between excitation and inhibition of BG output through these two pathways is important in the control of motor behavior (Wichmann and DeLong, 1998). It is generally accepted that striatal DA decreases BG output through a D1 DA receptor-mediated excitation of the direct pathway and a D2 DA receptor-mediated inhibition of the indirect pathway (Gerfen et al., 1990). A growing body of literature suggests that extrastriatal DA signaling may also modulate the output of the BG, thereby contributing to the fine-tuning of motor control. In addition to their nigro-striatal projection, neurons of the SNc also synthesize, store, and release DA from their cell bodies and ABBREVIATIONS: BG, basal ganglia; DA, dopamine; GABA, ␥-aminobutyric acid; SNr, substantia nigra pars reticulata; STN, subthalamic nucleus; SNc, substantia nigra pars compacta; PD, Parkinson’s disease; mGluR, metabotropic glutamate receptor; ACSF, artificial cerebrospinal fluid; IPSC, inhibitory postsynaptic current; EPSC, excitatory postsynaptic current; ANOVA, analysis of variance; PK, protein kinase; LY354740, (⫹)-2-aminobicyclo[3䡠1䡠0]-hexane-2,6-dicarboxylate monohydrate; SCH23390 hydrochloride, R-(⫹)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5tetrahydro-1H-3-benzazepine; SKF82958, (⫾)-6-chloro-7,8-dyhydroxy-3allyl-1-phenyl-2,3,4,5-tetra-hydro-1H-benzazepine; L-AP4, L-(⫹)-2-amino-4phosphonobutyric acid. 433 Downloaded from jpet.aspetjournals.org at ASPET Journals on May 5, 2017 ABSTRACT Recent findings have shown that dendritically released dopamine (DA) plays an important modulatory role in the substantia nigra pars reticulata (SNr). It is therefore possible that the loss of DA observed in Parkinson’s disease (PD) could hold important consequences for nigral function. Previously, we have shown that activation of presynaptically localized group II metabotropic glutamate receptors (mGluRs) inhibits excitatory transmission at the subthalamic nucleus (STN)-SNr synapse and that activation of presynaptically localized group III mGluRs decreases excitatory and inhibitory transmission in the SNr. To test the hypothesis that nigral DA can modulate mGluR function in the SNr, we performed whole-cell patch-clamp recordings from ␥-aminobutyric acidergic SNr neurons in slices obtained from rats that were acutely reserpinized. In slices obtained from reserpinized animals, the effect of group II mGluR activation by the selective agonist (⫹)-2-aminobicyclo[3䡠1䡠0]-hexane-2,6-dicarboxylate monohydrate (LY354740) (100 nM), but not group 434 Wittmann et al. Materials and Methods Electrophysiology. All experiments involving rats were carried out in accordance with the Guide for the Care and Use of Laboratory Animals. Whole-cell patch-clamp recordings were obtained under visual control as described previously (Bradley et al., 2000). Fifteento 18-day-old Sprague-Dawley rats were used for all patch-clamp studies. After decapitation, brains were rapidly removed and submerged in an ice-cold sucrose buffer: 187 mM sucrose, 3 mM KCl, 1.9 mM MgSO4, 1.2 mM KH2PO4, 20 mM glucose, and 26 mM NaHCO3, equilibrated with 95% O2, 5% CO2. Parasagittal slices (300 m in thickness) were made using a Vibraslicer (WPI, Sarasota, FL). Slices were transferred to a holding chamber containing normal ACSF: 124 mM NaCl, 2.5 mM KCl, 1.3 mM MgSO4, 1.0 mM NaH2PO4, 2.0 mM CaCl2, 20 mM glucose, and 26 mM NaHCO3, equilibrated with 95% O2/5% CO2. In all experiments, 5 M glutathione and 500 M pyruvate were included in the sucrose buffer and holding chamber to increase slice viability. Slices were transferred to the stage of a Hoffman modulation contrast microscope (Olympus Optical, Tokyo, Japan) and continually perfused with ACSF (⬃3 ml/min, 32°C). Neurons in the SNr were visualized with a 40⫻ water immersion lens. Patch electrodes were pulled from borosilicate glass on a vertical patch pipette puller (Narashige, Tokyo, Japan) and filled with 140 mM potassium gluconate, 10 mM HEPES, 10 mM NaCl, 0.6 mM EGTA, 0.2 mM NaGTP, and 2 mM MgATP, pH adjusted to 7.4 with 0.5 N KOH. Electrode resistance was 3 to 7 M⍀. GABA-ergic SNr neurons were identified according to previously established electrophysiological criteria (Richards et al., 1997). GABA-ergic neurons exhibited spontaneous repetitive firing, short duration action potentials, little spike frequency adaptation, and a lack of inward rectification, whereas dopaminergic neurons displayed no or low-frequency spontaneous firing, longer duration action potentials, strong spike frequency adaptation, and a pronounced inward rectification. All of the data presented in these studies are from neurons that fit the electrophysiological criteria of GABA-ergic neurons. Excitatory postsynaptic currents (EPSCs) and inhibitory postsynaptic currents (IPSCs) were evoked with a bipolar tungsten stimulation electrode (0.4 –12.0 A, every 30 s) placed within the cerebral peduncle rostral to and outside of the SNr. EPSCs were recorded from a holding potential of ⫺60 mV and bicuculline (20 M) was bath applied during all EPSC recordings to block inhibitory transmission. IPSCs were recorded at a holding potential of ⫺50 mV and 6-cyano2,3-dihydroxy-7-nitroquinoxaline (10 –20 M) and D(⫺)-2-amino-5phosphonopentanoic acid (10 –20 M) were present in the bath to block excitatory transmission. Kainate-evoked currents were recorded at a holding potential of ⫺60 mV, and tetrodotoxin (1 M) was present in the bath to block synaptic transmission. Kainate (100 M) was applied directly to the postsynaptic cell with a fast application system (Alagarsamy et al., 1999). Catecholamine Depletion. To deplete DA, reserpine (5 mg/kg i.p.) was administered acutely to the rats 1 to 1.5 h before sacrifice, and the brain slices were continually perfused with reserpine (10 –20 M) after the dissection. This acute treatment with reserpine induced a marked catalepsy in all animals (data not shown). The degree of depletion of DA in the SNr and SNc induced by similar treatment was described previously by histochemical and in vivo studies (Bjorklund and Lindvall, 1975; Elverfors and Nissbrandt, 1991; Heeringa and Abercrombie, 1995). Drugs and Drug Application. Drugs were made into stock solutions of 1 to 100 mM and diluted to the desired concentration in ACSF immediately before bath application to the slice. Antagonists were applied at least 15 min before application of agonists. Dopamine hydrochloride and (⫺)-quinpirole hydrochloride stocks were made in water containing sodium metabisulfite such that the final solution applied to the slice contained 50 M sodium metabisulfite. Reserpine stocks were made in glacial acetic acid at 5000 times its final concentration. Haloperidol hydrochloride stocks were made in 4 Downloaded from jpet.aspetjournals.org at ASPET Journals on May 5, 2017 dendrites (Bjorklund and Lindvall, 1975; Cheramy et al., 1981; Rice et al., 1997). By its actions on D2 autoreceptors located on SNc DA-ergic neurons, somatodendritically released DA can modulate SNc cell firing and subsequent DA release in the striatum (Santiago and Westerink, 1991). Furthermore, it has been shown that the dendrites of DA neurons in the SNc extend ventrally into the SNr (Bjorklund and Lindvall, 1975; Fallon and Loughlin, 1995) where they release DA (Geffen et al., 1976; Rice et al., 1997). This dendritically released DA is known to modulate GABA and glutamate release within the SNr (Abarca et al., 1995; Aceves et al., 1995; Radnikow and Misgeld, 1998) and influence SNr neurons (Martin and Waszczak, 1994; Huang and Walters, 1994; Martin and Waszczak, 1996). Recent studies suggest that the loss of SNc dopaminergic neurons observed in PD results in a loss of striatal DA and a concomitant increase in activity in the indirect pathway relative to the direct pathway (Wichmann and DeLong, 1998). The resulting increase in activity of glutamatergic neurons in the STN ultimately leads to a net increase in synaptic excitation of GABA-ergic projection neurons in the output nuclei and is believed to underlie the motor impairments characteristic of PD (DeLong, 1990). In addition to these effects on striatal function, recent in vivo studies indicate that loss of DA tone in the SNr also contributes to the symptoms of PD (Mayorga et al., 1999). We have previously reported that mGluRs modulate excitatory and inhibitory synaptic transmission in the SNr (Bradley et al., 2000; Wittmann et al., 2001). Furthermore, we have suggested that these receptors could provide possible novel therapeutic targets for the treatment of PD by providing a method of pharmacologically reducing excitatory drive through the indirect pathway. For example, we have demonstrated that group II mGluRs are presynaptically localized on STN terminals in the SNr and that activation of these receptors reduces excitatory synaptic transmission at the STN-SNr synapse (Bradley et al., 2000). Therefore, in the Parkinsonian state, an agonist of group II mGluRs could selectively reduce the increased excitatory drive through the indirect pathway. In contrast, we have shown that group III mGluRs are presynaptically located on excitatory and inhibitory terminals projecting to SNr neurons and that activation of these receptors decreases excitatory and inhibitory synaptic transmission in the SNr (Wittmann et al., 2001). This suggests that the group III mGluRs might be a less useful target for the treatment of PD because these effects on excitatory and inhibitory transmission would tend to cancel each other. DA can modulate the function of other G protein-coupled receptors (Ferre et al., 1992; Garcia et al., 1997; Chen et al., 2001), raising the interesting possibility that nigral DA can modulate the function of mGluRs in the SNr. Because DA depletion is the hallmark of PD, any alteration in mGluR pharmacology in a DA-depleted state would have important implications both for therapeutic targeting and our basic understanding of how these receptors modulate information flow through the BG. We therefore investigated the pharmacology of mGluR-mediated inhibition of synaptic transmission in the SNr of DA-depleted rats. Dopamine Modulates mGluR Function in SNr 435 parts of 1 N sodium hydroxide and 6 parts of 8.5% lactic acid. (S)-(⫺)-Sulpiride stocks were made in dimethyl sulfoxide at 5,000 to 10,000 its final concentration. L-AP4 stocks were made in 1 M equivalents of sodium hydroxide. LY354740, SCH23390 hydrochloride, (⫾)-SKF82958 hydrobromide, (⫺)-bicuculline methiodide, 6-cyano-2,3-dihydroxy-7-nitroquinoxaline disodium salt, D-(⫺)-2-amino-5-phosphonopentanoic acid, and kainic acid stock solutions were made in water. LY354740 was a gift from D. Schoepp and J. Monn (Eli Lilly, Indianapolis, IN). Other drugs were obtained from SigmaAldrich (St. Louis, MO), Alexis (San Diego, CA), and Tocris Cookson (Ballwin, MO). Data Analysis. Values are expressed as mean ⫾ S.E.M. Statistical comparisons between two groups were performed by using a Student’s t test. Statistical comparisons between several groups were performed by one-way ANOVA followed by Tukey’s post hoc comparisons. Results Fig. 1. In slices obtained from reserpinized animals, the suppression of EPSCs induced by the group II mGluR-selective agonist LY354740 is reduced in substantia nigra pars reticulata neurons. In control animals, application of LY354740 (100 nM) significantly and reversibly decreases EPSCs (n ⫽ 9; p ⬍ 0.01). A, example traces of evoked EPSCs in control animals before (predug), during (LY354740), and after (washout) brief bath application of LY354740. In reserpine-treated animals, this effect of LY354740 is significantly reduced. B, example traces of evoked EPSCs in reserpine-treated animals before, during, and after brief bath application of LY354740 (100 nM). C, bar graph showing the average effect of the selective group II mGluR agonist LY354740 (100 nM) in control animals and reserpine-treated animals. Each column represents the mean (⫾S.E.M.) of 9 and 12 experiments, respectively (ⴱⴱⴱ, p ⬍ 0.001; t test). group II mGluRs on excitatory synaptic transmission at STN-SNr synapses. To address this issue we investigated the effect of bath application of the group II mGluR-selective agonist LY354740 (100 nM) on kainate-evoked postsynaptic currents by fast application of 100 M kainate to the cell in the presence of tetrodotoxin (1 M) and the DA receptor antagonist haloperidol (20 M). We have shown previously that in control animals, activation of group II mGluRs has no effect on kainate-evoked currents (Bradley et al., 2000). Prior bath application of haloperidol (20 M) for 20 min had no Downloaded from jpet.aspetjournals.org at ASPET Journals on May 5, 2017 Dopamine Modulates the Group II mGluR-Mediated Inhibition of Excitatory Transmission in the Substantia Nigra Pars Reticulata. Previous studies have shown that group II mGluRs are presynaptically localized on glutamatergic terminals in the SNr and that activation of these receptors by group II mGluR-selective agonists decreases excitatory transmission in this nucleus by a presynaptic mechanism (Bradley et al., 2000). To determine whether endogenous nigral DA modulates this effect, we performed similar experiments in slices obtained from DA-depleted animals acutely treated with reserpine. As described previously (Bradley et al., 2000), 3-min bath application of 100 nM LY354740, a highly selective agonist of group II mGluRs, produced a significant depression of EPSCs at the STN-SNr synapse (54.5 ⫾ 4.5%; n ⫽ 9; p ⬍ 0.01; Figure 1, A and C). Interestingly, in slices obtained from DA-depleted animals the effect of LY354740 on EPSC amplitude was significantly decreased (19.9 ⫾ 4.1% inhibition of EPSCs; n ⫽ 12; p ⬍ 0.005; Figure 1, B and C). To further test the hypothesis that ambient DA modulates the function of presynaptic group II mGluRs at the STN-SNr synapse, we produced an acute block of DA receptors with selective DA antagonists in control slices. All antagonists were applied at least 20 to 30 min before bath application of LY354740. Bath application of 10 to 20 M haloperidol, which blocks both D1 and D2 receptors at this concentration, mimicked the effect of DA depletion, significantly reducing the depression of EPSCs induced by LY354740 (35.5 ⫾ 3.5%; n ⫽ 8; p ⬍ 0.01; Fig. 2, B and E). This effect of haloperidol was mimicked by the selective D1 receptor antagonist SCH23390 (20 –50 M) (30.4 ⫾ 2.8% inhibition of EPSCs; n ⫽ 7; p ⬍ 0.01; Fig. 2, C and E), but not by the D2-selective antagonist sulpiride (50 M) (45.7 ⫾ 4.3% inhibition of EPSCs; n ⫽ 6; p ⬎ 0.05; Fig. 2, D and E). Interestingly, higher concentrations of either haloperidol or the D1-selective antagonist SCH23390 showed a tendency to inhibit excitatory transmission (data not shown). Taken together, these data suggest that D1-like DA receptor activation controls presynaptic group II mGluR-mediated inhibition of excitatory transmission in the SNr. However, it is conceivable that DA receptor blockade or reserpine treatment unmask a postsynaptic group II mGluR-mediated effect that could counteract the presynaptic actions of group II mGluRs at this synapse. This postsynaptic effect could therefore lead to an overall decrease of the inhibitory effect of 436 Wittmann et al. Fig. 2. Prior bath application of DA receptor antagonists in slices obtained from control animals also decreases the effect of the group II mGluR-selective agonist LY354740 on EPSCs in the SNr. A to D, example traces of evoked EPSCs before (predrug) and during (LY354740) brief bath application of LY354740 alone (A) or in the presence of selective and nonselective DA antagonists (B–D). Prior bath application of the nonselective DA antagonist haloperidol (20 M) and the D1-selective antagonist SCH23390 (20 M) significantly decreases the effect LY354740 (100 nM) on EPSCs (B and C). Prior application of the D2-selective antagonist sulpiride (50 M) has no effect on the effect of LY354740 (D). E, bar graph showing the average effect of nonselective and selective DA antagonists on the effect induced by LY354740. Each column represents the mean (⫾S.E.M.) of data collected from six to nine cells (ⴱⴱ, p ⬍ 0.01; ANOVA and Tukey’s least significant difference). Downloaded from jpet.aspetjournals.org at ASPET Journals on May 5, 2017 effect on the ability of LY354740 (100 nM) to influence the amplitude of kainate-evoked currents (amplitude of kainateevoked current predrug, 243.3 ⫾ 79.1 pA; during application of 100 nM LY354740, 221.5 ⫾ 53.6 pA; n ⫽ 3; p ⬎ 0.5, paired t test). If the effect of reserpine treatment on the group II mGluRmediated inhibition of transmission at the STN-SNr synapse is due to a lack of activation of the D1 DA receptor, it should be possible to rescue the mGluR-mediated modulation in reserpinized animals by the application of DA, or selective D1 DA receptor agonists. We tested this hypothesis by bath applying DA agonists for at least 30 min before the application of LY354740 in slices obtained from reserpinized animals. Interestingly, bath application of 100 M DA restored the effect of the group II mGluR agonist from 19.9 ⫾ 4.1 to 42.1 ⫾ 9.5% inhibition of EPSCs (n ⫽ 5; p ⬍ 0.05; Fig. 3, B and E). Consistent with the antagonist pharmacology, this effect was mimicked by the selective D1-like DA receptor agonist SKF82958 (1 M) (46.2 ⫾ 2.7% inhibition of EPSCs; p ⬍ 0.05; n ⫽ 5; Fig. 3, C and E) but not by the D2-selective agonist quinpirole (10 M) (24.4 ⫾ 8.1% inhibition of EPSCs; n ⫽ 4; p ⬎ 0.05; Fig. 3, D and E). In addition, we found no potentiation of the effect of LY354740 (100 nM) on EPSCs in control rats induced by prior DA application (data not shown). Taken together, these data suggest that under normal conditions tonic activation of D1-like receptors by ambient DA maintains the group II mGluRs presynaptically localized at the STN-SNr synapse in an active state. Dopamine Does Not Modulate the Group III mGluRMediated Inhibition of Excitatory Transmission in the Substantia Nigra Pars Reticulata. Group III mGluRs are also presynaptically localized at the STN-SNr synapse, and activation of these receptors also decreases excitatory transmission in the SNr by a presynaptic mechanism (Wittmann et al., 2001). We therefore investigated whether ambient DA modulates the effect of group III mGluR-selective agonists on EPSCs. As described previously, the selective group III mGluR agonist L-AP4 (500 M) reduces EPSCs at the STNSNr synapse in slices obtained from normal animals (57.1 ⫾ 3.8%; n ⫽ 4; p ⬍ 0.05; Fig. 4, A and C). In contrast to the effect of reserpine treatment on group II mGluR-mediated inhibition of excitatory transmission, the effect of 500 M L-AP4 was not decreased in experiments performed in DAdepleted slices (68.5 ⫾ 5.2% inhibition of EPSCs; n ⫽ 5; p ⬎ 0.05; Fig. 4, B and C). Dopamine Modulates the Group III mGluR-Mediated Decrease of Inhibitory Synaptic Transmission in the Substantia Nigra Pars Reticulata. Group III mGluRs are also found presynaptically localized on GABA-ergic terminals in the SNr, and activation of these receptors by the group III mGluR-selective agonist L-AP4 inhibits IPSCs in Dopamine Modulates mGluR Function in SNr 437 Discussion We have shown previously that activation of mGluRs in the SNr produces an inhibition of synaptic transmission at both inhibitory and excitatory synapses. Activation of presynaptically localized group III mGluRs induces a decrease in inhibitory transmission (Wittmann et al., 2001), whereas activation of group II or group III mGluRs at the STN-SNr synapse produces a marked inhibition of excitatory transmission (Bradley et al., 2000; Wittmann et al., 2001). The present study provides evidence that ambient DA tonically modulates these mGluR functions in the SNr. In particular, DA depletion by reserpinization produces a marked decrease in the ability of group II mGluR agonists to inhibit excitatory transmission, and also in the ability of group III mGluR agonists to decrease inhibitory transmission. Interestingly, Fig. 3. Prior bath application of DA receptor agonists in slices obtained from reserpine-treated animals can restore the effect of the group II mGluR-selective agonist LY354740 on EPSCs in the SNr. A to D, example traces of evoked EPSCs before (predrug) and during (LY354740) brief bath application of LY354740 alone (A) or in the presence of selective and nonselective DA receptor agonists (B–D). Prior bath application of DA or the D1-selective agonist SKF82958 (1 M) significantly increases the effect LY354740 (100 nM) on EPSCs in reserpinized animals (B and C). Prior application of the D2-selective agonist quinpirole (10 M) has no effect on the effect of LY354740 (D). E, bar graph showing the average effect of nonselective and selective DA agonists on the effect induced by LY354740. Each column represents the mean (⫾S.E.M.) of data collected from 4 to 12 cells (ⴱ, p ⬍ 0.05; ANOVA and Tukey’s least significant difference). Downloaded from jpet.aspetjournals.org at ASPET Journals on May 5, 2017 GABA-ergic SNr neurons by a presynaptic mechanism (Wittmann et al., 2001). We therefore performed experiments to determine whether this effect of L-AP4 on IPSCs is modulated by ambient DA. As described previously, brief bath application of the group III mGluR-selective agonist L-AP4 (100 M) significantly decreased IPSC amplitude (80.7 ⫾ 3.8%; n ⫽ 8; p ⬍ 0.01; Fig. 5, A and C). Interestingly, this effect of 100 M L-AP4 was significantly decreased in slices from DA-depleted animals (47.6 ⫾ 9.2% inhibition of IPSCs; n ⫽ 6; p ⬍ 0.01; Fig. 5, B and C). To test whether acute blockade of DA receptors has the same effect as DA depletion we applied selective DA antagonists in control slices. Consistent with previous reports (Radnikow and Misgeld, 1998), we found that D1-selective antagonists decrease the amplitude of evoked IPSCs in the SNr (data not shown). We therefore applied all antagonists for at least 30 min to ensure that a stable baseline was reached. Haloperidol (20 M) mimicked the effect of DA depletion and significantly reduced the depression of IPSCs induced by L-AP4 (52.4 ⫾ 8.1% inhibition of IPSCs; n ⫽ 5; p ⬍ 0.05; Fig. 6, B and E). Interestingly, the effect of haloperidol was mimicked by both the D1-selective antagonist SCH23390 (20 M) (50.9 ⫾ 9.2% inhibition of IPSCs; n ⫽ 7; p ⬍ 0.05; Fig. 6, C and E), and the D2-selective antagonist sulpiride (20 M) (48.2 ⫾ 7.7% inhibition of IPSCs; n ⫽ 8; p ⬍ 0.05; Fig. 6, D and E), suggesting that both D1-like and D2-like receptors are involved in this modulation of group III mGluR function. Taken together, these data suggest that ambient DA modulates the function of presynaptically localized group II and III mGluRs on excitatory and inhibitory terminals in the SNr, respectively. Thus, in a Parkinsonian state, the loss of this modulatory control of excitatory and inhibitory inputs to the output neurons of the BG may add to the overall pathophysiological changes in the BG output. 438 Wittmann et al. the group III mGluR-induced inhibition of excitatory transmission is not effected by the reserpine treatment, indicating some level of receptor specificity. The effects of reserpine are mimicked in slices from control animals by the application of DA antagonists, and the mGluR-mediated effects are rescued in slices from reserpinized animals by the application of DA agonists. Therefore, tonic DA levels seem to be crucial in maintaining the mGluRs in an active state, and interactions Fig. 5. In slices obtained from reserpinized animals, the suppression of IPSCs induced by the group III mGluR-selective agonist L-AP4 is reduced in substantia nigra pars reticulata neurons. In control animals, application of L-AP4 (100 M) significantly and reversibly decreases IPSCs (n ⫽ 8; p ⬍ 0.01). A, example traces of evoked IPSCs in control animals before (predrug), during (L-AP4), and after (washout) brief bath application of L-AP4. In reserpine-treated animals, this effect of L-AP4 is reduced significantly. B, example traces of evoked IPSCs in reserpine-treated animals before, during, and after brief bath application of L-AP4 (100 M). C, bar graph showing the average effect of the selective group III mGluR agonist L-AP4 (100 M) in control animals and reserpine-treated animals. Each column represents the mean (⫾S.E.M.) of eight and six experiments, respectively (多多多, p ⬍ 0.01; t test). between these two transmitter systems may play a crucial role in the fine-tuning of synaptic function in the SNr. The present findings add to a growing body of literature suggesting that somatodendritically released DA is able to directly influence BG output. Behavioral studies have shown that local injection of DA agonists and antagonists into the SNr modulates locomotor activity in normal animals (Kelly et al., 1987; Trevitt et al., 2001) and in 6-hydroxy-dopaminelesioned rats (LaHoste and Marshall, 1990). In particular, Downloaded from jpet.aspetjournals.org at ASPET Journals on May 5, 2017 Fig. 4. Application of the group III mGluR-selective agonist L-AP4 (500 M) significantly and reversibly decreases EPSCs in the SNr (n ⫽ 4; p ⬍ 0.05). In contrast to the group II mGluR-mediated effect on EPSCs this effect is not affected in slices obtained from reserpinized animals. A, example traces of evoked EPSCs in control animals before (predug), during (L-AP4), and after (washout) brief bath application of L-AP4 (500 M). In slices obtained from reserpine-treated animals, this effect of L-AP4 is not reduced. B, example traces of evoked EPSCs in reserpinized slices before, during, and after brief bath application of L-AP4 (500 M). C, bar graph showing the average effect of the selective group III mGluR agonist L-AP4 (500 M) in control animals and reserpine-treated animals. Each column represents the mean (⫾S.E.M.) of four and five experiments, respectively (p ⬎ 0.05; t test). Dopamine Modulates mGluR Function in SNr 439 Fig. 6. Prior bath application of DA receptor antagonists in slices obtained from control animals also decreases the effect of the group III mGluR-selective agonist L-AP4 on IPSCs in the SNr. A to D, example traces of evoked IPSCs before (predrug) and during (L-AP4) brief bath application of L-AP4 alone (A) or in the presence of selective and nonselective DA antagonists (B–D). Prior bath application of the nonselective DA antagonist haloperidol (20 M), the D1-selective antagonist SCH23390 (20 M), and the D2-selective antagonist sulpiride (20 M) significantly decreases the effect L-AP4 (100 M) on IPSCs (B–D). E, bar graph showing the average effect of nonselective and selective DA antagonists on the effect induced by L-AP4. Each column represents the mean (⫾S.E.M.) of data collected from five to eight cells (ⴱ, p ⬍ 0.05; ANOVA and Tukey’s least significant difference). Downloaded from jpet.aspetjournals.org at ASPET Journals on May 5, 2017 the D1 DA receptor has been proposed as an important regulator of nigral function. Anatomical studies have shown that there is a dense localization of D1 receptors in the SNr (Yung et al., 1995). Activation of D1 receptors by local administration of agonists alters the firing activity of GABA-ergic SNr neurons measured in vivo (Waszczak, 1990; Martin and Waszczak, 1994). Activation of presynaptic D1 receptors at the nigrostriatal synapse induces an increase in GABA release both in vivo (Rosales et al., 1997) and in vitro (Aceves et al., 1995), and enhances inhibitory transmission (Radnikow and Misgeld, 1998) (but see also Miyazaki and Lacey, 1998). Furthermore, application of D1 antagonists has been shown to decrease IPSCs in slices, suggesting that presynaptic D1 DA receptors are tonically activated (Radnikow and Misgeld, 1998). The D1 DA receptor has also been implicated in modulation of excitatory transmission in the SNr. In vivo microdialysis experiments have shown that activation of the D1 DA receptor produces an increase in nigral glutamate efflux (Abarca et al., 1995), an effect likely mediated by the activation of receptors localized on STN terminals (Rosales et al., 1997). In addition, our finding that application of the D1selective antagonist SCH23390 showed a tendency to decrease EPSCs in the SNr is in agreement with these findings. Interestingly, activation of D1 receptors in the SNr has been shown to have antiparkinsonian actions (Mayorga et al., 1999) and has been proposed as a novel therapeutic treatment (Taylor et al., 1991; Williams et al., 1997). We previously suggested that agonists for group II mGluRs could provide novel therapeutic targets for the treatment of PD based on the findings that activation of presynaptic group II mGluRs at STN-SNr synapses decreases glutamate release and therefore could counteract the pathological increase in BG outflow observed in this disease. However, the present findings suggest that a loss of DA in the SNr leads to a decrease in group II mGluR function. Presynaptically localized group II mGluRs have been hypothesized to act as autoreceptors, providing a feedback control over the release of glutamate. Therefore, pathological loss of nigral DA may exacerbate the hyperactivation of the BG output nuclei observed in PD. The effect of DA on group II mGluR function is mediated by activation of D1 but not D2 receptors. Therefore, activation of D1 receptors at the STN-SNr synapse may help reduce transmission at this synapse by maintaining feedback control of glutamate release by group II mGluRs. It is of interest that the effects of DA depletion where restricted to the group II mGluRs at the STN-SNr synapse, yet effected group III mGluR function at inhibitory synapses. This indicates that both group II and III mGluRs can be modulated by the activation of DA receptors, yet some degree of specificity must be imparted by differences in cellular biochemistry. The basic mechanism by which activation of DA receptors suppresses group II mGluR function on excita- 440 Wittmann et al. References Abarca J, Gysling K, Roth RH, and Bustos G (1995) Changes in extracellular levels of glutamate and aspartate in rat substantia nigra induced by dopamine receptor ligands: in vivo microdialysis studies. Neurochem Res 20:159 –169. Aceves J, Floran B, Sierra A, and Mariscal S (1995) D-1 receptor mediated modulation of the release of gamma-aminobutyric acid by endogenous dopamine in the basal ganglia of the rat. Prog Neuropsychopharmacol Biol Psychiatry 19:727–739. Alagarsamy S, Marino MJ, Rouse ST, Gereau RW, Heinemann SF, and Conn PJ (1999) Activation of NMDA receptors reverses desensitization of mGluR5 in native and recombinant systems. Nat Neurosci 2:234 –240. Bjorklund A and Lindvall O (1975) Dopamine in dendrites of substantia nigra neurons: suggestions for a role in dendritic terminals. Brain Res 83:531–537. Bradley SR, Marino MJ, Wittmann M, Rouse ST, Awad H, Levey AI, and Conn PJ (2000) Activation of group II metabotropic glutamate receptors inhibits synaptic excitation of the substantia nigra pars reticulata. J Neurosci 20:3085–3094. Cai Z, Saugstad JA, Sorensen SD, Ciombor KJ, Zhang C, Schaffhauser H, Hubalek F, Pohl J, Duvoisin RM, and Conn PJ (2001) Cyclic AMP-dependent protein kinase phosphorylates group III metabotropic glutamate receptors and inhibits their function as presynaptic receptors. J Neurochem 78:756 –766. Chen JF, Moratalla R, Impagnatiello F, Grandy DK, Cuellar B, Rubinstein M, Beilstein MA, Hackett E, Fink JS, Low MJ, et al. (2001) The role of the D2 dopamine receptor (D2R) in A2A adenosine receptor (A2AR)-mediated behavioral and cellular responses as revealed by A2A and D2 receptor knockout mice. Proc Natl Acad Sci USA 98:1970 –1975. Cheramy A, Leviel V, and Glowinski J (1981) Dendritic release of dopamine in the substantia nigra. Nature (Lond) 289:537–542. DeLong MR (1990) Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13:281–285. Elverfors A and Nissbrandt H (1991) Reserpine-insensitive dopamine release in the substantia nigra. Brain Res 557:5–12. Fallon JH and Loughlin SE (1995) Substantia nigra, in The Rat Nervous System (Paxinos G ed) pp 215–237, Academic Press, San Diego. Ferre S, Fuxe K, von Euler G, Johansson B, and Fredholm BB (1992) Adenosinedopamine interactions in the brain. Neuroscience 51:501–512. Fuxe K, Ferre S, Zoli M, and Agnati LF (1998) Integrated events in central dopamine transmission as analyzed at multiple levels. Evidence for intramembrane adenosine A2A/dopamine D2 and adenosine A1/dopamine D1 receptor interactions in the basal ganglia. Brain Res Rev 26:258 –273. Garcia M, Floran B, Arias-Montano JA, Young JM, and Aceves J (1997) Histamine H3 receptor activation selectively inhibits dopamine D1 receptor-dependent [3H]GABA release from depolarization-stimulated slices of rat substantia nigra pars reticulata. Neuroscience 80:241–249. Geffen LB, Jessell TM, Cuello AC, and Iversen LL (1976) Release of dopamine from dendrites in rat substantia nigra. Nature (Lond) 260:258 –260. Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ Jr, and Sibley DR (1990) D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science (Wash DC) 250:1429 –1432. Grofova I, Deniau JM, and Kitai ST (1982) Morphology of the substantia nigra pars reticulata projection neurons intracellularly labeled with HRP. J Comp Neurol 208:352–368. Heeringa MJ and Abercrombie ED (1995) Biochemistry of somatodendritic dopamine release in substantia nigra: an in vivo comparison with striatal dopamine release. J Neurochem 65:192–200. Huang KX and Walters JR (1994) Electrophysiological effects of SKF 38393 in rats with reserpine treatment and 6-hydroxydopamine-induced nigrostriatal lesions reveal two types of plasticity in D1 dopamine receptor modulation of basal ganglia output. J Pharmacol Exp Ther 271:1434 –1443. Kamiya H and Yamamoto C (1997) Phorbol ester and forskolin suppress the presynaptic inhibitory action of group-II metabotropic glutamate receptor at rat hippocampal mossy fiber synapse. Neuroscience 80:89 –94. Kelly E, Jenner P, and Marsden CD (1987) Comparison of changes in locomotor activity with striatal homovanillic acid and 3,4-dihydroxyphenylacetic acid concentrations following the bilateral intranigral injection of dopamine agonist drugs in rats. J Pharm Pharmacol 39:196 –202. LaHoste GJ and Marshall JF (1990) Nigral D1 and striatal D2 receptors mediate the behavioral effects of dopamine agonists. Behav Brain Res 38:233–242. Macek TA, Schaffhauser H, and Conn PJ (1998) Protein kinase C and A3 adenosine receptor activation inhibit presynaptic metabotropic glutamate receptor (mGluR) function and uncouple mGluRs from GTP-binding proteins. J Neurosci 18:6138 – 6146. Martin LP and Waszczak BL (1994) D1 agonist-induced excitation of substantia nigra pars reticulata neurons: mediation by D1 receptors on striatonigral terminals via a pertussis toxin-sensitive coupling pathway. J Neurosci 14:4494 – 4506. Martin LP and Waszczak BL (1996) Dopamine D2, receptor-mediated modulation of the GABA-ergic inhibition of substantia nigra pars reticulata neurons. Brain Res 729:156 –169. Mayorga AJ, Trevitt JT, Conlan A, Gianutsos G, and Salamone JD (1999) Striatal and nigral D1 mechanisms involved in the antiparkinsonian effects of SKF 82958 (APB): studies of tremulous jaw movements in rats. Psychopharmacology 143:72– 81. Milligan G (1993) Mechanisms of multifunctional signalling by G protein-linked receptors. Trends Pharmacol Sci 14:239 –244. Miyazaki T and Lacey MG (1998) Presynaptic inhibition by dopamine of a discrete component of GABA release in rat substantia nigra pars reticulata. J Physiol (Lond) 513:805– 817. Radnikow G and Misgeld U (1998) Dopamine D1 receptors facilitate GABAA synaptic currents in the rat substantia nigra pars reticulata. J Neurosci 18:2009 – 2016. Rice ME, Cragg SJ, and Greenfield SA (1997) Characteristics of electrically evoked somatodendritic dopamine release in substantia nigra and ventral tegmental area in vitro. J Neurophysiol 77:853– 862. Richards CD, Shiroyama T, and Kitai ST (1997) Electrophysiological and immunocytochemical characterization of GABA and dopamine neurons in the substantia nigra of the rat. Neuroscience 80:545–557. Rocheville M, Lange DC, Kumar U, Patel SC, Patel RC, and Patel YC (2000) Receptors fro dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science (Wash DC) 288:154 –157. Rosales MG, Martinez-Fong D, Morales R, Nunez A, Flores G, Gongora-Alfaro JL, Downloaded from jpet.aspetjournals.org at ASPET Journals on May 5, 2017 tory terminals, and group III mGluR function on inhibitory terminals is not presently understood. One possible mechanism is suggested by the recent findings that the function of presynaptic mGluRs is tightly regulated by protein kinases. It has been shown that protein kinase (PK) C inhibits the function of several group II and III mGluR subtypes on glutamatergic terminals in a variety of brain regions (Kamiya and Yamamoto, 1997; Macek et al., 1998). Furthermore, recent studies have shown that several presynaptic group II and III mGluR subtypes are phosphorylated by PKA. This phosphorylation inhibits receptor coupling to G proteins and therefore decreases their ability to activate effector systems (Schaffhauser et al., 2000; Cai et al., 2001), an effect that can be elicited by the stimulation of endogenous Gs-coupled receptors (Cai et al., 2001). If D1 receptor and D2 receptor stimulation by ambient DA was able to inhibit PKA or PKC in the SNr, and therefore keep group II and III mGluRs from being phosphorylated, this might explain the loss of mGluR function in the reserpinized state. Recent studies have shown that D1 receptors and D2 receptors can couple to multiple effector pathways or to different pathways in different cells (Milligan, 1993). Traditionally, D1 receptors are thought to activate adenylyl cyclase, whereas D2 receptor stimulation decreases adenylyl cyclase activity. This could explain the effect of D2 receptor blockade and DA depletion on group III mGluR function on inhibitory terminals in the SNr. Whether D1 receptor activation inhibits adenylyl cyclase in SNr terminals, or activates another effector system that influences mGluR function in these terminals requires further investigation. Another possible mechanism by which activation of DA receptors could suppress group II and III mGluR function on excitatory and inhibitory terminals could be a direct receptor-receptor interaction at these terminals. Direct receptorreceptor interactions and formation of heterodimers has been proposed for other G protein-coupled receptors such as somatostatin 5 receptors and D2 DA receptors or for A2A receptors and D2 DA receptors in the BG (Fuxe et al., 1998; Rocheville et al., 2000). Finally, we cannot exclude the possibility of a postsynaptic action of DA on DA receptors localized on SN neurons, releasing a retrograde messenger that maintains presynaptic mGluR functions under normal circumstances. In summary, these studies suggest that ambient, probably dendritically released DA modulates the function of presynaptic group II and III mGluRs in the SNr. DA depletion induces a decrease in the inhibitory actions of presynaptic group II mGluRs at the glutamatergic STN-SNr synapses and the inhibitory action of presynaptic group III mGluRs on GABA-ergic transmission in the SNr. Thus, the loss of nigral DA may contribute to the overall pathophysiology of PD. This interdependence of different families of G protein-coupled receptors has important implications for therapeutic targeting, and reinforces the notion that it is crucial to validate any therapeutic approach in an animal model that closely approximates the disease state. Dopamine Modulates mGluR Function in SNr Floran B, and Aceves J (1997) Reciprocal interaction between glutamate and dopamine in the pars reticulata of the rat substantia nigra: a microdialysis study. Neuroscience 80:803– 810. Santiago M and Westerink BH (1991) The regulation of dopamine release from nigrostriatal neurons in conscious rats: the role of somatodendritic autoreceptors. Eur J Pharmacol 204:79 – 85. Schaffhauser H, Cai Z, Hubalek F, Macek TA, Pohl J, Murphy TJ, and Conn PJ (2000) cAMP-dependent protein kinase inhibits mGluR2 coupling to G-proteins by direct receptor phosphorylation. J Neurosci 20:5663–5670. Taylor JR, Lawrence MS, Redmond DE, Elsworth JD, Roth RH, Nichols DE, and Mailman RB (1991) Dihydrexidine, a full dopamine D1 agonist, reduces MPTPinduced parkinsonism in monkeys. Eur J Pharmacol 199:389 –391. Trevitt JT, Carlson BB, Nowend K, and Salamone JD (2001) Substantia nigra pars reticulata is a highly potent site of action for the behavioral effects of the D1 antagonist SCH 23390 in the rat. Psychopharmacology 156:32– 41. Waszczak BL (1990) Differential effects of D1 and D2 dopamine receptor agonists on substantia nigra pars reticulata neurons. Brain Res 513:125–135. 441 Wichmann T and DeLong MR (1998) Models of basal ganglia function and pathophysiology of movement disorders. Neurosurg Clin N Am 9:223–236. Williams M, Wright S, and Lloyd GK (1997) Improved therapies for Parkinson’s disease: life beyond dopamine D2/D3 receptor agonists. Trends Pharmacol Sci 18:307–310. Wittmann M, Marino MJ, Bradley SR, and Conn PJ (2001) Activation of group III mGluRs inhibits GABA-ergic and glutamatergic transmission in the substantia nigra pars reticulata. J Neurophysiol 85:1960 –1968. Yung KK, Bolam JP, Smith AD, Hersch SM, Ciliax BJ, and Levey AI (1995) Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience 65:709 –730. Address correspondence to: Dr. P. Jeffrey Conn, Senior Director, Neuroscience, Merck Research Laboratories, Merck & Co., Inc., 770 Sumneytown Pike, P.O. Box 4, WP 46-300, West Point, PA 19486-0004. E-mail: [email protected] Downloaded from jpet.aspetjournals.org at ASPET Journals on May 5, 2017